Abstract
Melanin is major factor that determines skin color as well as one of the defense systems that prevent the UV-induced damage. In case of abnormal concentration of melanin, skin diseases or problems occur such as albinism, leukoplakia, melasma, freckles, moles, and lentigo. With the lifespan of humans has been extended, importance of ‘life quality’ has been increased. White and clean skin is very important part of the satisfaction of appearance, especially for Asia women. The aim of this study was to find an anti-melanogenesis activity for which the aerial part of Pueraria thunbergiana can be utilized based on the increase in demands for cosmetics, particularly natural products. We demonstrated anti-pigmentation effects of aerial part of P. thunbergiana by measuring melanin content and through staining in the B16F10 melanoma cell line. The aerial part of P. thunbergiana decreased tyrosinase activity significantly in B16F10 cell cultures, while there is no direct effect on enzyme in cell-free conditions. To define the mechanisms, real-time PCR, western blot, glucosidase activity and antioxidant activity assay were implemented. As results, we demonstrated that aerial part of P. thunbergiana has anti-melanogenesis activity via two mechanisms. One is downgrading microphthalmia-associated transcription factor by activating Akt/GSK-3β. Consequently, transcription of tyrosinase and tyrosinase-related protein 1 is decreased. Another is interrupting maturation of tyrosinase through inhibiting α-glucosidase. Furthermore, aerial part of P. thunbergiana showed great efficacy on pigmentation in vivo. These results suggest that aerial part of P. thunbergiana can be used as an anti-melanogenic agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pueraria thunbergiana, also known as the kudzu, is a species of climbing plant belonging to the Leguminosae family. The root and flower of P. thunbergiana, used in traditional medicine, have various medicinal properties [15, 33, 35, 36, 38]. However, the vine of P. thunbergiana is commonly discarded as a waste product and presents an environmental problem. The vine grows by climbing adjoining structures and trees, and destroys forests and landscape because of its weight and fast rate of growth. In some countries, P. thunbergiana is considered among the invasive species and seen as a threat to the ecosystem, with its management exacting a high cost, both financially and in terms of manpower [9, 16].
Melanin is a major factor that determines skin color, as well as one of the defense systems that prevent UV-induced skin damage. Abnormal concentrations of melanin manifest as skin diseases or problems, such as albinism, leukoplakia, melasma, freckles, moles, and lentigo. Skin-whitening agents are commonly applied for treating pigmentation and pigmentary diseases. Because of an increasing interest in herbs, many studies focused on discovering novel natural skin-whitening agents that are currently underway [30]. We investigated, therefore, whether the aerial part of P. thunbergiana, which is currently considered a waste product, has potentially useful skin-whitening effects.
Melanin is synthesized in melanocytes by enzyme-related mechanism, such as tyrosinase, tyrosinase-related protein 1 (TRP1) and tyrosinase-related protein 2 (TRP2). The first step of melanogenesis is a transformation of l-tyrosine into 3,4-dihydroxyphenylalanine (l-DOPA), and this l-DOPA is converted into DOPA quinine [28]. Then from this product, melanin is synthesized by subsequent transformation. First two ensuing stages, regulated by tyrosinase, are rate-limiting steps because the rest of the melanin synthesis process can go along naturally at a physiological pH [11]. Since tyrosinase is the key enzyme of melanogenesis, many whitening agents are targeting the tyrosinase by various mechanisms such as interference with tyrosinase catalytic activity directly, inhibition of mRNA expression, interruption of tyrosinase maturation, and acceleration of tyrosinase degradation [1].
Transcription levels of melanogenic genes are regulated by microphthalmia-associated transcription factor (MITF) [32]. MITF is expressed by that kind of stimulating factors. On the other hand, mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinases (ERK) and Akt/glycogen synthase kinase-3β (GSK-3β) signals pathways are involved in the down-regulation of melanogenesis by phosphorylating MITF [18, 34]. The phosphorylated MITF decreases its activity, binding to tyrosinase promoter site, and eventually degrades. Thereby, activation of MEK/ERK or Akt/GSK-3 inhibits melanin synthesis [14, 18].
The melanogenesis is regulated by nutritional and hormonal factors. l-tyrosine and l-DOPA are not only substrates but also bioregulator for melanogenesis. Regulation for melanocyte function of l-tyrosinase and l-DOPA can be mediated through specific receptors or without receptors [28]. In addition, there are complex multiple hormonal stimulators such as MSH, adrenocorticotropic hormone (ACTH), endorphin and stem cell factor (SCF) and inhibitors such as serotonin, melatonin, dopamine, acetylcholine and melanin-concentrating hormone (MCH) [25].
Since in vivo study is also important regarding the transmembrane or transdermal permeation, it was undertaken using cream formulations. There are differences between epidermal and follicular melanogenesis although basic features such as existence of melanocyte and melanosome are common. The follicular melanogenesis depends on anagen stage of hair cycle, whereas epidermal melanogenesis is continuous and follicular melanocyte is more influenced by aging [26]. Consequently, melanin-generating hairless mice irradiated UVB were used for in vivo study [31]. When skin exposes to UV, α-melanocyte-stimulating hormone (α-MSH), one of the important hormones which modulate pigmentation, is produced as a response [5].
Materials and methods
Reagents
Antibiotic–antimycotic, bovine serum albumin (BSA), Dulbecco’s modified Eagle’s medium (DMEM), and fetal bovine serum were purchased from Life Technologies (Carlsbad, CA, USA). Acarbose, dimethyl sulfoxide (DMSO), 3,4-dihydroxy-l-phenylalanine (l-DOPA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), ethylenediaminetetraacetic acid (EDTA), α-glucosidase (EC 3.2.1.20, from baker’s yeast, 77 U/mg), LY294002, α-melanocyte-stimulating hormone (α-MSH), mushroom tyrosinase and ρ-Nitrophenyl-α-d-glucopyranoside were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fontana–Masson staining kit was obtained from IHCWORLD (Woodstock, MD, USA). Phosphate-buffered saline (PBS) was obtained from Bioworld Technology, Inc. (BioWorld, USA). easy-Blue™ Total RNA extraction kit was purchased from Intron Biotechnology, Inc. (iNtRON Biotechnology, Seongnam, Korea). TaqMan® RNA-to-Ct™ 1-Step Kits were purchased from Applied Biosystems (USA). RIPA buffer was obtained from Biosesang Inc. (Seongnam, Korea). Protease inhibitor cocktail tablets were purchased from Roche Diagnostics (Switzerland). Antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA) and Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). ECL reagent was purchased from GE Healthcare.
Extraction of plant material
Aerial parts of P. thunbergiana were collected from Jinan, Jeonbuk, Korea, in November 2010, and extracted by the Hanpoong Pharm and Foods Company (Hanpoong Pharm. CO., Ltd.). Briefly, dried and pulverized materials (2 kg) were boiled with 2 L of distilled water and a range of ethanol concentrations (0, 30, 70, and 95 %) for 3 h. The solvent was then removed under reduced pressure in a rotary evaporator (N-1000S, EYELA, Japan) to yield a water extract (439.5 g), 30 % ethanolic extract (409.2 g), 70 % ethanolic extract (436.7 g), and a 95 % ethanolic extract (284.8 g).
The respective extracts were suspended with distilled water, and then partitioned with ethyl acetate (EtOAc). The EtOAc and aqueous fractions were independently evaporated under reduced pressure at 60 °C, and the extracts completely dried. Aqueous fractions, with increasing ethanol concentration in the initial extraction step, are referred to as extract Nos. 1–4, while the organic (EtOAc) fraction extracts are referred to as Nos. 5–8 (Fig. 1).
High-performance liquid chromatography analysis
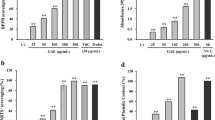
The isoflavone content of the extracts was quantitated by high-performance liquid chromatography (HPLC; Waters 2695 system, Waters Co., Milford, MA, USA) using a CAPCELLPAK UG 120 250 × 4.6 mm, 5-µm column (Shiseido Co., Tokyo, Japan). Separation and quantification of isoflavones were achieved at 30 °C using a solvent gradient from solution A, a 10 % aqueous methanol solution with 2 % acetic acid, to solution B, a 98 % aqueous methanol solution with 2 % acetic acid, at a flow rate of 1 mL/min. Peaks were detected by measuring absorbance at 260 nm. The presence of isoflavones was confirmed by comparing the observed peaks with the retention time of the corresponding standards (Wako Chemical Co., Osaka, Japan). The chromatogram of total isoflavones is shown in Fig. 2, and the chemical structures of each isoflavone are indicated in Fig. 3. The isoflavone aglycone, and glycoside concentrations in each extract are listed in Table 1.
Cell culture and treatment with extracts
Murine B16F10 melanoma cells purchased from ATCC (Manassas, VA, USA) were cultured in DMEM with 10 % (v/v) fetal bovine serum and 1 % (v/v) antibiotic–antimycotic. The cells were incubated in a humidified incubator under 5 % CO2 at 37 °C. Aqueous fractions are dissolved in DDW and EtOAc fractions are dissolved in DMSO. Controls of B16F10 cells were added DDW or DMSO at the same concentration as in the treated cells.
Cell viability assay
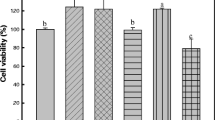
Cell proliferation was evaluated by performing the MTT assay. The cells (3 × 103 cells/well) were seeded into a 96-well plate and incubated for 24 h; the cells were subsequently exposed to the P. thunbergiana extracts at a range of concentrations (0, 5, 10, 50, 100, 500, and 1,000 μg/mL) for 2 days. Thereafter, serum-free MTT medium was added to each well to the final concentration of 1 mg/mL, and incubation performed for 2–4 h at 37 °C. The MTT medium was removed from the wells, and DMSO was added; thereafter, the plate was placed on a shaker for 5 min. Absorbance was read at 540 nm using a microplate spectrophotometer (SpectraMax 190, USA).
Melanin content
The B16F10 cells (3 × 104 cells/well) were seeded in a 6-well plate and exposed to α-MSH (100 nM) for 1 day. The treatment was performed with a combination of α-MSH (100 nM) and P. thunbergiana extracts for 2 days. The cells were harvested by trypsinization and washed twice with PBS and alcohol. 2 × 105 cells were dissolved in 200 μL of 1 N NaOH with 10 % DMSO at 90 °C for 1 h. The resulting melanin concentration was quantified by measuring the absorbance at 475 nm.
Mushroom tyrosinase activity
Reactions were performed in potassium phosphate buffer (pH 6.81). Mushroom tyrosinase solution was prepared by dissolving 25,000 units in 6 mL of 0.1 mM potassium phosphate buffer and adding 2 mL of distilled water. l-DOPA solution (0.01 %) in distilled water was used as the enzyme substrate. A mixture of 160 μL of buffer, 20 μL of substrate, 10 μL of P. thunbergiana extract, and 10 μL of enzyme was added to a 96-well plate. Tyrosinase activity was quantified by measuring absorbance at 475 nm after 2 min. Kojic acid was used as a positive control. The data are expressed as mean ± SD.
Cellular tyrosinase assay
Another method of measuring tyrosinase activity was repeated since there are complex regulations of tyrosinase activation [24]. The B16F10 cells were exposed to α-MSH (100 nM) for 1 day and treated with a combination of extracts and α-MSH for 2 days. The pellet was obtained by trypsinization and washed with PBS. The cells were lysed with 0.1 M sodium phosphate buffer (pH 6.8) containing 5 mM EDTA, 1 % Triton X-100, and 0.1 % phenylmethylsulfonyl fluoride (PMSF) in ice for 30 min. After centrifugation of the lysate at 15,000 rpm for 30 min at 4 °C, cellular tyrosinase activity was measured in the resulting supernatant. Enzyme activity was normalized to protein concentration, as determined by Bradford assay. The cellular tyrosinase and 0.1 % l-DOPA reaction were performed in 0.1 M sodium phosphate buffer at 37 °C for 1 h. Tyrosinase activity was quantified by measuring the absorbance at 475 nm.
Real-time PCR
Quantification of selected genes transcript by real-time PCR was performed using a TaqMan® RNA-to-Ct™ 1-Step Kit according to the manufacturer’s instructions. The mRNA was extracted with easy-Blue™ Total RNA extraction kit and quantified by Nano Drop. Relative ratio of a target gene expression was calculated with the ΔC t method.
Western blot analysis
After treatment with a range of concentrations (0, 10, 50, and 100 μg/mL) of extracts, the B16F10 cells were washed with PBS, and lysed by RIPA buffer in ice to get the protein. Next, 20–50 μg of proteins was subjected to electrophoresis on 10–15 % sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE). Following electrophoresis, the proteins were transferred to nitrocellulose membranes, and the membranes were blocked with 5 % skimmed milk or 5 % BSA in PBS with 0.1 % Tween 20 (PBST). Then, the proteins were probed using antibodies. The immunoblots were developed and visualized using an enhanced chemiluminescence (ECL) detection system (Amersham Biosciences, Piscataway, NJ, USA). The western blot was imaged using the Chemi Documentation Imaging System and quantified using densitometric program (Image J). GAPDH and β-actin were used as an internal control.
Glucosidase activity assay
Inhibition activities of α-glucosidase were evaluated according to the chromogenic method described by McCue et al. [17]. Yeast α-glucosidase (0.5 unit/mL) 20 µL, 0.1 M phosphate buffer (pH 6.9) 120 and 10 µL of test sample dissolved in DMSO at various concentrations were mixed in a microplate well. After incubating for 15 min at 37 °C, 20 µL of ρ-Nitrophenyl-α-d-glucopyranoside (5 mM) was added to substrates and incubated for an additional 15 min. The reaction was terminated by 80 µL of 0.2 M sodium carbonate (Na2CO3) solution. The absorbance was measured at 405 nm. Acarbose was used as positive control. Each experiment was conducted in triplicate, and the IC50 values of samples were calculated.
Antioxidant activity
DPPH was dissolved in methanol to a 0.1 mM concentration, and mixed with the diluted P. thunbergiana extracts (0, 10, 50, and 100 μg/mL) in a 96-well plate in a 1:1 ratio. Reaction was allowed to proceed in the dark for 30 min, and the absorbance was measured at 520 nm. Ascorbic acid was used as a standard. The data are expressed as mean ± SD.
Animals
Six-week-old male hairless mice, Hos: HRM2, were purchased from Hoshino Laboratory Animals Inc. (Yashio, Saitama, Japan). The animals were housed in a SPF animal facility room at 23 ± 1 °C and 50 ± 10 % relative humidity with 12-h light/dark cycle with free access to standard commercial diet and water. Creams containing 1 or 3 % of No.6 extracts were used for assessing in vivo anti-melanogenesis efficacy. There are five groups of mice in the animal model; control group (applied cream base, CON), UVB-exposed control group (UVB + applied cream base, UVB), positive control group (UVB + applied 1 % kojic acid cream, kojic acid), experiment group 1 [UVB + applied 1 % No.6 cream, No.6 (1 %)] and experiment group 2 [UVB + applied 3 % No.6 cream, No.6 (3 %)].
In vivo skin pigmentation determinations
The treatments were performed once per every day since 3 days before UVB irradiation by topically applying 100 mg of each cream on the dorsal skin of the animal. The mice were anesthetized with a ketamine–xylazine mixture (2 mL/kg, i.p., respectively) and after 30 min to absorb the cream, exposed to UVB at 100, 150 and 200 mJ/cm2 once per day for each 5 days, respectively. Colors of skin were determined using a DSM II ColorMeter (Cortes Technology). The results were expressed as L* values (value of 0 indicates black, 100 indicates white). At the 19 day, mice were sacrificed and the dorsal skins were obtained. The specimens were used for Fontana–Masson staining. ΔL* were calculated as follows:
Fontana–Masson staining
The B16F10 melanoma cells were cultured and treated in a chamber slide. After removal of the medium, the cells were fixed with 4 % formalin for 20 min and washed twice by PBS containing 0.1 % Triton X-100. The cells were stained with the Fontana–Masson staining kit according to manufacturer’s instructions.
Each specimen was soaked in formaldehyde to fix. Then, paraffin processing and embedding were carried out. The tissue blocks were sectioned at 4 μm. The skins were stained with Fontana–Masson staining kit.
Briefly, the B16F10 cells or specimens were incubated in pre-warmed FM silver nitrate solution, toned in gold chloride solution, and soaked in 5 % sodium thiosulfate solution. Finally, counterstaining was performed with the Nuclear Fast Red Solution.
Statistical analysis
All results obtained from at least three independent experiments were combined and analyzed using the Student’s t test. All values were expressed as the mean ± SD. The acceptable level of significance was established at p values <0.05
Results
The aerial part of P. thunbergiana hinders melanogenesis in B16F10 cells
To evaluate the whitening effect of the aerial part of P. thunbergiana, B16F10 melanoma cells were treated with the extracts at a range of concentrations that showed no adverse effects on cell viability (Fig. 4). Melanin synthesis was induced by α-MSH in B16F10 cells and 100 μg/mL, the highest concentration without cytotoxicity, of samples were treated for screening. α-MSH is one of the factors that stimulate pigmentation in mammals [12]. The aqueous fractions of P. thunbergiana extracts had no effects on melanogenesis, but the EtOAc-soluble fractions of the extracts inhibited α-MSH-induced melanin synthesis more effectively than did kojic acid, a whitening agent used as a positive control (Fig. 5). When the data are expressed as a percentage normalized to the non-treated cells, the cells treated with extracts No.6 (EtOAc-soluble fraction of the 30 % EtOH extract), and No.7 (EtOAc-soluble fraction of the 30 % EtOH extract) showed 21.17 and 48.20 % of melanin contents, respectively, lower than that shown by the non-treated cells.
Concentration-dependent effects of aerial part of P. thunbergiana on B16F10 cell viability. Cytotoxicity of P. thunbergiana extracts in B16F10 melanoma cells was evaluated by treating the cells treated with aqueous or EtOAc fraction of extracts for 48 h. Cell viability was determined using the MTT assays, and results are expressed as percent viability relative to untreated cells
Effect of aerial part of P. thunbergiana on melanogenesis in B16F10 cells. B16F10 cells were cultured, and following adherence, the cells were treated with α-MSH. After 1 day, the cells were incubated with combinations of α-MSH and kojic acid or P. thunbergiana extract Nos. 1–8. Melanogenesis was quantified 2 days later, and the results expressed relative to controls. **p < 0.01, and ***p < 0.001 compared to cells stimulated with α-MSH alone
Further examination showed that the treatment of the cells with the EtOAc-soluble fraction of P. thunbergiana extracts reduced melanogenesis in a dose-dependent manner (Fig. 6). The melanogenesis-suppressing activity of extract Nos. 6 and 7, most significant samples in collective assessment, was confirmed visually by Fontana–Masson staining (Fig. 7). Accumulation of melanin was observed in α-MSH-treated B16F10 cells and treatment with P. thunbergiana extracts down-regulated the pigmentation in the cells. At the highest concentration used, extract No.6 inhibited melanogenesis to the baseline (non-treated cells) level.
Effect of EtOAc fractions on melanogenesis in B16F10 cells. B16F10 cells were cultured, and following adherence, the cells were treated with α-MSH. The cells were treated with a combination of α-MSH with kojic acid, arbutin, or a range of concentrations of extract Nos. 5–8 (5, 10, 50, and 100 μg/mL). The results were expressed relative to untreated cells. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to α-MSH-stimulated cells
Optical images (magnification ×400) showing melanin content with Fontana–Masson staining in B16F10 cells. After 24 h of α-MSH pre-incubation, B16F10 cells were treated with a combination of α-MSH and extract No. 6 or 7, arbutin, or kojic acid for 48 h. Pigmentation observed upon Fontana–Masson staining (magnification ×400)
The aerial part of P. thunbergiana indirectly inhibits tyrosinase activity
The process of melanogenesis is regulated by an intracellular enzymatic cascade. To determine if the aerial part of P. thunbergiana directly affects tyrosinase, the key enzyme for melanin synthesis, we used mushroom and cellular tyrosinase activity assays. Unlike kojic acid, P. thunbergiana extracts did not exhibit any inhibitory effect on the tyrosinase activity in cell-free condition (Fig. 8). Interestingly, the cellular tyrosinase in B16F10 cells stimulated by α-MSH was significantly inhibited by treatment with P. thunbergiana extracts in a dose-dependent manner (Fig. 9). In the case of extract No.6, cellular tyrosinase activity declined to levels lower than those in the non-treated cells (Fig. 9a).
Comparison of cell-free tyrosinase activity of aerial part of P. thunbergiana. Reactions were performed in potassium phosphate buffer with l-DOPA and mushroom tyrosinase. After 2 min, tyrosinase activity was assessed by measuring the absorbance at 475 nm and expressed relative to the controls. Kojic acid was used as a positive control
Inhibitory effects of aerial part of P. thunbergiana on cellular tyrosinase activity. B16F10 cells were pre-cultured with α-MSH for 24 h, and incubated for 48 h more in a medium containing several concentrations (10, 50, and 100 µg/mL) of extract No. 6 (a) or No. 7 (b). The cellular tyrosinase activity was measured and the results are expressed relative to controls. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to α-MSH-stimulated cells
Effects on expression of mRNA and protein of tyrosinase
We conducted real-time PCR assay for inquiry about effects of aerial part of P. thunbergiana on mRNA of tyrosinase (Fig. 10a). Transcriptions of tyrosinase were significantly attenuated compared to transcription of α-MSH-stimulated cells. Moreover, we found dose-dependent manner effects of Nos. 6 and 7. Especially, tyrosinase expressions of B16F10 cells treated with 100 μg/mL were lower than the level of non-treated cells. The decrease of tyrosinase activity was further confirmed by western blot assay (Fig. 10b). There were double bands, and these bands indicate degrees of maturation of tyrosinase. Upper bands for glycosylated tyrosinase represent the mature form, while other bands for unglycosylated tyrosinase represent the immature form. Tyrosinase becomes the active form after being glycosylated. Treatment of Nos. 6 and 7 inhibited glycosylation of tyrosinase.
Effects of aerial part of P. thunbergiana on mRNA and proteins expression of tyrosinase. B16F10 cells were treated with α-MSH and No. 6 or No. 7 for the indicated concentration. mRNA levels (a) and expression levels (b) of tyrosinase were estimated. GAPDH was used as an internal standard. c–f Quantitation of the western blots by using GAPDH as the loading control. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to α-MSH-stimulated cells. ### p < 0.001 compared to nonstimulated cells
Inhibitory effects on α-glucosidase
To identify how aerial part of P. thunbergiana hinders maturation of tyrosinase, the study about α-glucosidase activity was conducted. Briefly, we demonstrated that both Nos. 6 and 7 have α-glucosidase inhibitory activity. The extracts inhibited α-glucosidase more effectively than acarbose, the synthetic inhibitor of α-glucosidase (Fig. 11). Dose–response curves of activity were obtained, and the IC50 values were calculated. No. 6 dramatically reduced α-glucosidase activity with IC50 of 349.70 μg/mL. The IC50 of No. 7 was the smallest with 213.93 μg/mL which is about ten times lower than acarbose with 2,010.34 μg/mL.
Effects on expression of mRNA and protein of TRP1
To find out effects of aerial part of P. thunbergiana on TRP1, we examined the mRNA and protein levels after treating a range of concentration of Nos. 6 and 7 (0, 10, 50, and 100 μg/mL) on α-MSH-stimulated B16F10 cells. Transcriptions of TRP1 were significantly attenuated in a dose-dependent manner (Fig. 12a). Expressions of TRP1 were significantly decreased by Nos. 6 and 7 (Fig. 12b).
Effects of aerial part of P. thunbergiana on mRNA and proteins expression of TRP1. B16F10 cells were treated with α-MSH and No. 6 or No. 7 for the indicated concentration. mRNA levels (a) and expression levels (b) of TRP1 were estimated. GAPDH was used as an internal standard. c–d Quantitation of the western blots by using GAPDH as the loading control. *p < 0.05 and ***p < 0.001 compared to α-MSH-stimulated cells. ### p < 0.001 compared to nonstimulated cells
Aerial part of P. thunbergiana inhibits MITF expression through the activation of Akt/GSK-3β signaling pathway
We found that the aerial Part of P. thunbergiana inhibits transcription of tyrosinase and TRP1 through MITF down-regulation. As shown in Fig. 13, expression of MITF was decreased by treatments with Nos. 6 and 7. We assessed phosphorylation of GSK-3β and ERK by implementing western blot since activated Akt/GSK-3β and MEK/ERK signals degrade MITF. Phosphorylation of GSK-3β was significantly enhanced by Nos. 6 and 7, respectively. p-ERK was not significantly influenced.
Effects of aerial part of P. thunbergiana on expression of melanogenesis-related proteins. B16F10 cells were treated with α-MSH and No. 6 or No. 7 for the indicated concentration. Western blot assay were performed to estimate and expression levels of total MITF, p-ERK and p-GSK-3β. Protein loading amounts were confirmed by GAPDH expression. b–g Quantitation of the western blots by using GAPDH as the loading control. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to α-MSH-stimulated cells
Therefore, we examined melanin formations in B16F10 cells co-treated with No. 6 or 7 and LY294002 as Akt/GSK-3β-specific inhibitor. As shown in Fig. 14, treatment of LY294002 induced, and treatment of Nos. 6 and 7 inhibited melanin synthesis compared with control. The melanin production, suppressed by Nos. 6 and 7, was restored by LY294002. These results suggested that the aerial part of P. thunbergiana activates Akt/GSK-3β.
Effects of aerial part of P. thunbergiana on regulation of Akt/GSK-3β signal. Melanin contents were evaluated. Cells were pretreated in the absence (−) or presence (+) of LY294002 (20 µM) for 1 h and then cultured without (−) or with (+) 50 µg/mL of No. 6 (a) and 7 (b) for 48 h. ***p < 0.001 compared to sample-treated only
Evaluation of the in vivo depigmenting activity of aerial part of P. thunbergiana
To survey the potentiality as human whitening agent, the depigmenting activity of aerial part of P. thunbergiana was estimated by in vivo system using melanin-procession hairless mice for 19 days. As shown in Fig. 15, the degrees of pigmentation were measured as L* value representing the lightness of the color. The values of UVB-exposed control group (UVB) have significantly decreased compared with control group (CON) since 6 day. The treatments with No. 6 creams mitigated pigmentation, respectively. The L* values were significantly higher than UVB-exposed control group (UVB) in No. 6 1 % cream-applied animals [No. 6 (1 %)] after 9 day. Besides, in No. 6 3 % cream-applied animals [No. 6 (3 %)], the values were not decreased by UVB irradiation and have been significantly higher than the values of UVB-exposed control (UVB) since 8 day. Furthermore, the values have increased at comparing with control group (CON) since 14 day (Fig. 15a). For positive control, the calculated UVB-induced decreases of L* values were alleviated by application of kojic acid 1 % cream (Kojic acid) (Fig. 15b).
Effects of aerial part of P. thunbergiana on pigmentation in UV-irradiated animal. a Melanin possessing hairless mice were treated with 100 mg of cream base, kojic acid 1 % cream, No. 6 1 and 3 % cream on dorsal skins every day. UVB irradiation was performed according to the indicated schedules. The lightness (L* value) of skin was measured before applying cream on each day. b ΔL* values of each animal were calculated; values of days 17, 18, 19 minus values of days 2, 3, 4. Data represent mean ± SEM (n = 3). # p < 0.05, ## p < 0.01, and ### p < 0.001 compared with CON. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with UVB group. c Microscopic images of skin sections. Melanogenesis of dorsal skin was highlighted by Fontana–Masson staining. Arrows indicate pigmentation
Microscopic observation of the epidermis
Effects of aerial part of P. thunbergiana on pigmentation in UVB-irradiated mice were confirmed by Fontana–Masson staining (Fig. 15c). In the UVB-exposed-control epidermis, an induction of melanogenesis was observed compared to controls. In mice applied with No. 6 (1 and 3 %), melanogenesis was reduced.
Discussion
Currently, numerous compounds are used for skin whitening, such as arbutin, hydroquinone, and kojic acid. However, there is growing interest in finding alternative agents since the existing ones possess mutagenic properties and can induce skin disorders or low depigmenting activity [8, 20].We investigated, therefore, the effects of the aerial part of P. thunbergiana, an overlooked resource, on melanin synthesis. Both aqueous and EtOAc fractions of the extracts obtained using a range of EtOH densities (0, 30, 70, and 95 %) were studied.
We found that the EtOAc fraction significantly reduced melanin synthesis in the B16F10 melanin-processing cell lines (Fig. 5) without causing cytotoxicity (Fig. 4). The P. thunbergiana extracts exhibited dose-dependent inhibition, with extract Nos. 6 and 7 (30 and 70 % ethanol, respectively) proving to be excellent melanogenesis inhibitors (Fig. 6). In addition, anti-pigmentation effects of the aerial part of P. thunbergiana were clearly demonstrated by the intracellular melanin accumulation observed using staining techniques (Fig. 7).
A number of mechanisms exist for the regulation of melanogenesis [10]. The most common target for depigmenting agents is tyrosinase, the rate-limiting enzyme of melanogenesis. Whitening ingredients such as ascorbic acid, thio-containing compounds, phenolic compounds, and kojic acid inhibit tyrosinase enzyme activity [6]. In this study, we have established that the aerial part of P. thunbergiana reduces tyrosinase activity through its effect on cellular tyrosinase in α-MSH-treated B16F10 cells (Fig. 9). However, the aerial part of P. thunbergiana had no direct inhibitory effects on tyrosinase (Fig. 8). These results indicate that the anti-melanogenesis activity of the aerial part of P. thunbergiana is involved in superior levels regulating tyrosinase enzyme such as transcription, translation, and maturation [23].
To understand the mechanisms of inhibitory effects on melanogenesis, we examined the effects on mRNA and protein expression of tyrosinase and TRP1, enzymes involved in melanogenesis. Aerial part of P. thunbergiana inhibits transcription and protein levels of tyrosinase and TRP1 (Figs. 10, 12).
Transcription of tyrosinase and TRP1 is promoted by MITF [2, 32]. We found out that MITF was down-regulated by aerial part of P. thunbergiana (Fig. 13). It was reported that the activation of Akt/GSK-3β and MEK/ERK signaling pathway downgrades activity and stability of MITF and leads to degradation [13]. Accordingly, we investigated the phosphorylation of GSK-3β and ERK. Phosphorylation of GSK-3β was increased significantly by aerial part of P. thunbergiana in a dose-dependent manner. p-ERK had no change (Fig. 13). In addition, we found that melanin formations, which were inhibited by aerial part of P. thunbergiana, recovered with the Akt/GSK-3β-specific inhibitor LY294002 (Fig. 14). These results mean that aerial part of P. thunbergiana inhibits MITF through modulation of the Akt/GSK-3β pathway, not MEK/ERK.
Tyrosinase, the key enzyme of melanogenesis, is one of the glycoprotein which completes its maturation by processing N-glycosylation. Initial tyrosinase is glycosylated when translated polypeptide chain translocates into the endoplasmic reticulum (ER). After trimming by α-glucosidase, glycosylated tyrosinase interacts with chaperones and folds. Folded tyrosinase is completely matured via acquisition of two Cu2+ in Golgi and transported to melanosome [4]. Our data revealed that aerial part of P. thunbergiana reduces tyrosinase maturation (Fig. 10) by inhibiting α-glucosidase (Fig. 11).
UV radiation is a strong inducer of oxidative stress, contributing to skin pigmentation. Therefore, antioxidants can reduce melanogenesis [3, 19]. In this study, we detected antioxidant activity in the extracts of the aerial part of P. thunbergiana using DPPH assay, with the EtOAc fractions exhibiting stronger antioxidant properties than the aqueous fractions (data not shown). The EtOAc-soluble fractions showed remarkable scavenging effects compared to those of ascorbic acid, a well-known antioxidant [22]. The antioxidant activity could be regarded as one of the reasons that the aerial part of P. thunbergiana has anti-melanogenesis activity. Further study about antioxidant effect will be needed, because there are many mechanism linking antioxidant activity and melanin synthesis [27, 29].
Taken together, the molecular and biological mechanisms underlying the inhibition of melanogenesis are mediated via complex pathways (Fig. 16). We confirmed that there are a lot of isoflavones in aerial part of P. thunbergiana (Table 1). The isoflavones are potent antioxidant agents and major active compounds of several Leguminosae plants (Fig. 3) [7, 21]. In practice, a study about the effect of genistein on melanogenesis by inhibitory effect on α-glucosidase has been reported [37]. Thus, it could be anticipated that the isoflavones are major components of aerial part of P. thunbergiana for anti-melanogenic effect. To define active compound, additional studies such as column chromatography and nuclear magnetic resonance (NMR) are going to be progressed.
The multiple mechanisms of anti-melanogenesis effects of the aerial part of P. thunbergiana. The aerial part of P. thunbergiana has multi-action mediating inhibition of melanogenesis. The suppression in expression of tyrosinase and TRP1 is associated with MITF which is downgraded by Akt/GSK-3β signal. Maturation of tyrosinase is interrupted by inhibitory effect on α-glucosidase of the aerial part of P. thunbergiana. Antioxidant activity also inhibits melanogenesis
Furthermore, we demonstrated that topical spread of cream contained extract of aerial part of P. thunbergiana can inhibit pigmentation in vivo study (Fig. 15). Application of aerial part of P. thunbergiana reduced UVB-induced-pigmentation, and the effect was more dramatical than kojic acid. In case of treatment of aerial part of P. thunbergiana 3 % contained cream, the skin lightness was higher than control that was not exposed to UVB. Furthermore, by Fontana–Masson staining study, it was identified that melanin distribution of epidermis induced by UVB was inhibited by aerial part of P. thunbergiana (Fig. 15c). These results of our study suggest that aerial part of P. thunbergiana can be used as a skin-whitening agent.
References
Ando H, Kondoh H, Ichihashi M, Hearing VJ (2007) Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J Invest Dermatol 127(4):751–761. doi:10.1038/sj.jid.5700683
Aoki H, Moro O (2002) Involvement of microphthalmia-associated transcription factor (MITF) in expression of human melanocortin-1 receptor (MC1R). Life Sci 71(18):2171–2179
Bolling BW, Blumberg JB, Chen CY (2009) Extraction methods determine the antioxidant capacity and induction of quinone reductase by soy products in vitro. Food Chem 116(1):351–355. doi:10.1016/j.foodchem.2009.01.087
Branza-Nichita N, Petrescu AJ, Negroiu G, Dwek RA, Petrescu SM (2000) N-glycosylation processing and glycoprotein folding-lessons from the tyrosinase-related proteins. Chem Rev 100(12):4697–4712
Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M (1996) Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim Biophys Acta 1313(2):130–138
Chang TS (2009) An updated review of tyrosinase inhibitors. Int J Mol Sci 10(6):2440–2475. doi:10.3390/Ijms10062440
Dixit AK, Bhatnagar D, Kumar V, Chawla D, Fakhruddin K, Bhatnagar D (2012) Antioxidant potential and radioprotective effect of soy isoflavone against gamma irradiation induced oxidative stress. J Funct Foods 4(1):197–206. doi:10.1016/j.jff.2011.10.005
Findlay GH, de Beer HA (1980) Chronic hydroquinone poisoning of the skin from skin-lightening cosmetics. A South African epidemic of ochronosis of the face in dark-skinned individuals. S Afr Med J 57(6):187–190
Forseth IN, Innis AF (2004) Kudzu (Pueraria montana): history, physiology, and ecology combine to make a major ecosystem threat. Crit Rev Plant Sci 23(5):401–413. doi:10.1080/07352680490505150
Gillbro JM, Olsson MJ (2011) The melanogenesis and mechanisms of skin-lightening agents–existing and new approaches. Int J Cosmet Sci 33(3):210–221. doi:10.1111/j.1468-2494.2010.00616.x
Halaban R, Patton RS, Cheng E, Svedine S, Trombetta ES, Wahl ML, Ariyan S, Hebert DN (2002) Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J Biol Chem 277(17):14821–14828. doi:10.1074/jbc.M111497200
Hunt G, Todd C, Cresswell JE, Thody AJ (1994) Alpha-melanocyte stimulating hormone and its analogue Nle4DPhe7 alpha-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci 107(Pt 1):205–211
Jang JY, Kim HN, Kim YR, Choi WY, Choi YH, Shin HK, Choi BT (2011) Partially purified components of Nardostachys chinensis suppress melanin synthesis through ERK and Akt signaling pathway with cAMP down-regulation in B16F10 cells. J Ethnopharmacol 137(3):1207–1214. doi:10.1016/j.jep.2011.07.047
Kono M, Dunn IS, Durda PJ, Butera D, Rose LB, Haggerty TJ, Benson EM, Kurnick JT (2006) Role of the mitogen-activated protein kinase signaling pathway in the regulation of human melanocytic antigen expression. Mol Cancer Res 4(10):779–792. doi:10.1158/1541-7786.MCR-06-0077
Lim DW, Lee C, Kim IH, Kim YT (2013) Anti-inflammatory effects of total isoflavones from Pueraria lobata on cerebral ischemia in rats. Molecules 18(9):10404–10412. doi:10.3390/molecules180910404
Lindgren CJ, Castro KL, Coiner HA, Nurse RE, Darbyshire SJ (2013) The biology of invasive alien plants in Canada. 12. Pueraria montana var.lobata(Willd.) Sanjappa & Predeep. Can J Plant Sci 93(1):71–95. doi:10.4141/cjps2012-128
Mccue P, Kwon YI, Shetty K (2005) Anti-amylase, anti-glucosidase and anti-angiotensin I-converting enzyme potential of selected foods. J Food Biochem 29(3):278–294. doi:10.1111/j.1745-4514.2005.00020.x
Oka M, Nagai H, Ando H, Fukunaga M, Matsumura M, Araki K, Ogawa W, Miki T, Sakaue M, Tsukamoto K, Konishi H, Kikkawa U, Ichihashi M (2000) Regulation of melanogenesis through phosphatidylinositol 3-kinase-Akt pathway in human G361 melanoma cells. J Invest Dermatol 115(4):699–703. doi:10.1046/j.1523-1747.2000.00095.x
Panich U, Kongtaphan K, Onkoksoong T, Jaemsak K, Phadungrakwittaya R, Thaworn A, Akarasereenont P, Wongkajornsilp A (2010) Modulation of antioxidant defense by Alpinia galanga and Curcuma aromatica extracts correlates with their inhibition of UVA-induced melanogenesis. Cell Biol Toxicol 26(2):103–116. doi:10.1007/s10565-009-9121-2
Parvez S, Kang M, Chung HS, Cho C, Hong MC, Shin MK, Bae H (2006) Survey and mechanism of skin depigmenting and lightening agents. Phytother Res 20(11):921–934. doi:10.1002/ptr.1954
Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA (1997) Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res 26(1):63–70
Sharma OP, Bhat TK (2009) DPPH antioxidant assay revisited. Food Chem 113(4):1202–1205. doi:10.1016/j.foodchem.2008.08.008
Shin YJ, Han CS, Lee CS, Kim HS, Ko SH, Hwang SK, Ko SG, Shin JW, Ye SK, Chung MH (2010) Zeolite 4A, a synthetic silicate, suppresses melanogenesis through the degradation of microphthalmia-associated transcription factor by extracellular signal-regulated kinase activation in B16F10 melanoma cells. Biol Pharm Bull 33(1):72–76
Slominski A, Pruski D (1993) Melatonin inhibits proliferation and melanogenesis in rodent melanoma cells. Exp Cell Res 206(2):189–194. doi:10.1006/excr.1993.1137
Slominski A, Tobin DJ, Shibahara S, Wortsman J (2004) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84(4):1155–1228. doi:10.1152/physrev.00044.2003
Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ (2005) Hair follicle pigmentation. J Invest Dermatol 124(1):13–21. doi:10.1111/j.0022-202X.2004.23528.x
Slominski A, Wortsman J, Tobin DJ (2005) The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J 19(2):176–194. doi:10.1096/fj.04-2079rev
Slominski A, Zmijewski MA, Pawelek J (2012) l-tyrosine and l-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res 25(1):14–27. doi:10.1111/j.1755-148X.2011.00898.x
Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD (2012) Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol 212(v, vii):1–115
Smit N, Vicanova J, Pavel S (2009) The hunt for natural skin whitening agents. Int J Mol Sci 10(12):5326–5349. doi:10.3390/ijms10125326
Song K, An SM, Kim M, Koh JS, Boo YC (2011) Comparison of the antimelanogenic effects of p-coumaric acid and its methyl ester and their skin permeabilities. J Dermatol Sci 63(1):17–22. doi:10.1016/j.jdermsci.2011.03.012
Tachibana M (2000) MITF: a stream flowing for pigment cells. Pigment Cell Res 13(4):230–240
Wong KH, Li GQ, Li KM, Razmovski-Naumovski V, Chan K (2011) Kudzu root: traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J Ethnopharmacol 134(3):584–607. doi:10.1016/j.jep.2011.02.001
Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, Fisher DZ, Fisher DE (2000) c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev 14(3):301–312
Xiong Y, Yang Y, Yang J, Chai H, Li Y, Jia Z, Wang Z (2010) Tectoridin, an isoflavone glycoside from the flower of Pueraria lobata, prevents acute ethanol-induced liver steatosis in mice. Toxicology 276(1):64–72. doi:10.1016/j.tox.2010.07.007
Yamazaki T, Hosono T, Matsushita Y, Kawashima K, Someya M, Nakajima Y, Narui K, Hibi Y, Ishizaki M, Kinjo J, Nohara T (2002) Pharmacological studies on Puerariae Flos. IV: effects of Pueraria thomsonii dried flower extracts on blood ethanol and acetaldehyde levels in humans. Int J Clin Pharmacol Res 22(1):23–28
Yang E-S, Hwang J-S, Choi H-C, Hong R-H, Kang S-M (2008) The effect of genistein on melanin synthesis and in vivo whitening. Korean J Microbiol Biotechnol 36(1):72–81
Yasuda T, Endo M, Kon-no T, Kato T, Mitsuzuka M, Ohsawa K (2005) Antipyretic, analgesic and muscle relaxant activities of Pueraria isoflavonoids and their metabolites from Pueraria lobata Ohwi-a traditional Chinese drug. Biol Pharm Bull 28(7):1224–1228
Acknowledgments
This paper was supported by Wonkwang University in 2014.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Han, E., Chang, B., Kim, D. et al. Melanogenesis inhibitory effect of aerial part of Pueraria thunbergiana in vitro and in vivo. Arch Dermatol Res 307, 57–72 (2015). https://doi.org/10.1007/s00403-014-1489-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-014-1489-z