Abstract
Introduction
The periosteum is a readily available tissue at the hamstring harvest site that could be utilized to enhance graft healing and prevent tunnel widening without additional cost or morbidity. This study aimed to compare graft healing using magnetic resonance imaging (MRI) and functional clinical outcome scores in a matched cohort of patients who underwent anterior cruciate ligament (ACL) reconstruction with hamstring autografts with or without periosteal augmentation.
Material and methods
Forty-eight patients who underwent ACL reconstruction (ACLR) were prospectively enrolled: 25 with standard ACLR (ST-ACLR) and 23 with periosteal augmented grafts (PA-ACLR). The same surgical techniques, fixation methods, and postoperative protocol were used in both groups. Signal-to-noise quotient (SNQ), graft healing at the bone-graft interface, graft signal according to the Howell scale, and femoral tunnel widening were evaluated using MRI after 1 year of follow-up. International knee documentation score (IKDC), Lysholm, Tegner activity scale, and visual analog scale for pain were used for functional evaluation at a minimum of 2 years postoperative.
Results
The mean SNQ of the proximal part of the graft was 9.6 ± 9.2 and 2.9 ± 3.3 for the ST-ACLR and PA-ACLR groups, respectively (P = 0.005). The mean femoral tunnel widening was 30.3% ± 18.3 and 2.3% ± 9.9 for the ST-ACLR, PA-ACLR groups, respectively (P < 0.001). Complete graft tunnel healing was observed in 65% and 28% of cases in the PA-ACLR and ST-ACLR groups, respectively. Both groups showed marked improvements in functional scores, with no statistically significant differences.
Conclusion
Periosteal wrapping of hamstring tendon autografts is associated with better graft healing and maturation and lower incidence of femoral tunnel widening based on MRI analysis 1 year after ACL reconstruction. However, patient-reported outcomes and measured laxity were similar between the two groups at 2 years follow up.
Trial registration
Trail registration number: PACTR202308594339018, date of registration: 1/5/2023, retrospectively registered at the Pan African Clinical Trial Registry (pactr.samrc.ac.za) database.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hamstring tendon autografts are widely used for anterior cruciate ligament (ACL) reconstruction [1]. However, slower graft-tunnel incorporation, graft maturation, and tunnel widening remain some of the biggest concerns with hamstring autografts compared with BTB autografts [2,3,4]. Several studies have investigated different methods for enhancing graft-tunnel healing to increase osteointegration, including the use of bone morphogenic proteins, platelet-derived growth factors, transforming growth factor-b1 (TGF-b1), bone substitutes, and periosteum autografts [5,6,7].
The periosteum is a natural, readily available, osteogenic tissue that consists of two histologically different layers: the outer fibrous layer and the inner cambium layer that comprise mesenchymal progenitor cells or undifferentiated precursor cells capable of differentiating into either osteoblasts or chondrocytes depending on the culture environment [7]. The clinical outcomes of ACL reconstruction with periosteum-augmented hamstring tendon grafts have been previously reported, with encouraging results regarding functional and radiological parameters [8, 9]. Most of these studies used transtibial femoral drilling and two small free periosteal flaps sutured to the graft at the femoral and tibial tunnel apertures [7,8,9].
The present study aimed to compare the radiological findings using MRI scan obtained at 12 months postoperative and functional clinical outcome scores in a matched cohort of patients who underwent ACL reconstruction with hamstring autografts, either with or without periosteal augmentation with a minimum clinical follow-up period of 24 months. It was hypothesised that patients who underwent ACL reconstruction with periosteal augmentation would have better graft-tunnel healing, maturation, and less tunnel widening, with subsequently better functional outcomes at a 2-year follow up.
Material and methods
This prospective cohort study was conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by the local ethical committee (ethical approval code: 36264PR207/1/17). In addition, the study received approval from our institutional review board and all patients provided informed consent for participation. Patients who underwent primary single-bundle ACL reconstruction between February 2017 and January 2020 were prospectively enrolled. All procedures were performed by two surgeons (A. H. and S. F.) at the same institution using an autologous hamstring graft.
The inclusion criteria were (1) Skeletally mature male patients aged between 18 and 50 years, (2) Isolated ACL injury confirmed clinically (positive Lachman, pivot shift, and anterior drawer tests) and radiologically on MRI, (3) Healthy contralateral knee, (4) Absence of previous injuries in the affected knee, (5) Intact or repaired menisci at the time of surgery, (6) Low grade pivot shift (Grade 0–2) detected on examination under anesthesia, (7) Posterior tibial slope (PTS) measured on lateral plain radiograph < 12, (8) ACL graft diameter more than 8 mm, and (9) Patients with less than 3 months of interval between injury and surgery. Further intraoperative criteria included preserved tibial remnants (Crain type I–III) after graft implantation covering at least 25% of the intraarticular graft length. The following exclusion criteria were applied: (1) Posterior cruciate ligament (PCL), lateral and medial collateral ligament injuries higher than grade 2; (2) Cartilage damage or osteoarthritic changes (Outerbridge classification > 2), and (3) Meniscal deficiencies or tears that underwent partial meniscectomy.
In total, 59 patients were enrolled in the study. Patients were assigned consecutively into two groups in a 1:1 balanced allocation: a periosteal augmentation ACL reconstruction (PA-ACLR) and standard ACL reconstruction group (ST-ACLR) groups. Four patients were lost during the follow-up period, four patients did not undergo follow-up MRI examination, and three patients (1.7%) had to undergo a second surgery for varied reasons (graft rupture, cyclops resection, and meniscal retear) prior to the final clinical assessment and, therefore, 11 patients were excluded (7 patients from PA-ACLR group and 4 patients from ST-ACLR group). Ultimately, 48 patients were included in the analysis: 23 patients in the (PA-ACLR) group and 25 patients in the (ST-ACLR) group. The patient demographics are presented in Table 1.
Surgical technique
After receiving regional anesthesia, the patients were placed in a supine position on the operating table with the leg flexed to 90º and kept in position with foot and lateral support. Both the gracilis and semitendinosus tendons were harvested using an open stripper, and by the aid of a sharp scalpel and a periosteal elevator the distal ends of the hamstring grafts were harvested and freed from the tibial attachment together with an attached periosteal flap measuring approximately 1.5 × 3 cm (Fig. 1).
The grafts were tripled or quadrupled according to their length and diameter; as a result, the final graft was ≥ 8 mm in diameter and ≥ 8 cm in length. The periosteal layer was reflected to cover the proximal 1.5–3 cm of the graft entering the femoral tunnel, with the cambium layer directed outwards to face the femoral bony tunnel and sutured with vicryl 2–0. The femoral end of the graft was fixed to a fixed-loop EndoButton (Smith & Nephew, Andover, MA, USA) (Fig. 2).
The femoral tunnel was drilled using an inside-out transportal approach followed by tibial tunnel creation at the center of the ACL footprint. Tibial remnants were preserved as much as possible. The graft was passed intraarticularly from the tibial side, and the button was flipped, secured at the lateral femoral cortex, and checked using an image intensifier. After passage of the graft, the tibial remnant length covering the graft was measured and recorded. The knee was cycled multiple times and the graft was fixed on the tibial side with maximal manual tension at 20° of knee flexion and neutral rotation using a bioabsorbable screw (Smith & Nephew, Andover, MA, USA), with a diameter similar to that of the tibial tunnel. The wounds were closed in a standard fashion, and an intraarticular drain was placed.
Postoperative rehabilitation
Postoperative rehabilitation protocol was the same for all patients in both groups, the knee was immobilized in a brace in full extension for the first 2 weeks; meanwhile, aided partial weight-bearing was allowed as tolerated. Isometric quadriceps and straight leg-raising exercises were initiated as early as possible to achieve full extension. After 2 weeks, movement within a protected range of motion (ROM) of 0° to 90° was permitted. At 6 weeks, free ROM was allowed with no restrictions. After that period, under the supervision of a physiotherapist, rehabilitation and conditioning program was initiated and patients were allowed to resume sports at 6 months; pre-injury sports activities were limited until 9 months later.
Methods of evaluation
-
a. Subjective scores:
All patients participating in the study completed the International Knee Documentation Committee (IKDC), Lysholm, and Tegner activity scale subjective questionnaires preoperatively and at the final follow-up. The visual analogue scale (VAS) was used to measure pain intensity at the harvest site at 3, 6, and 12 months.
-
b. Objective evaluation:
Knee stability was graded and recorded using the Lachman and pivot shift tests preoperatively and at a minimum of 2 years postoperatively. The Lachman test was graded as 0 (negative), 1 (positive with firm endpoint), or 2 (positive with no endpoint), and the pivot shift test as 0 (absent), 1 (glide), 2 (jerk), or 3 (subluxation). Radiologically, the anterior tibial translation (ATT) was measured using a weight-bearing single-leg stance lateral radiograph at 20º of knee flexion at a minimum of 2 years postoperatively. ATT is defined as the distance between two lines parallel to the posterior tibial cortex, the first tangent to the posterior aspect of the medial tibial plateau and the second tangent to the posterior femoral condyles.
-
c. MRI evaluation:
Graft ligametization was evaluated using MRI performed 1 year postoperatively. The following parameters were evaluated: signal-to-noise quotient (SNQ) [10, 11], femoral tunnel aperture widening, graft healing to the femoral tunnel walls based on Ge et al. classification [12], and graft maturity according to the Howell scale [13].
Knee MRI was performed after 1 h of rest. A 3-T MRI unit (Magnetom Skyra, with a 15-channel phased-array send/receive knee coil; Siemens AG Healthcare) was utilized; a protocol that allowed multi-planar T2-weighted reconstructions parallel and perpendicular to the graft was used. Additional MR sequences included oblique sagittal 2D T2 sequencing along the intraarticular segment of the graft, T2 mapping, sagittal and axial 2-dimensional (2D) short tau inversion recovery (STIR), and coronal 2D proton density.
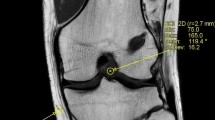
The MRI images were imported into a DICOM viewer (Radi-Ant DICOM Viewer 2020.2.2; Medixant Company, Poznan, Poland). According to a previously validated measuring technique using multi-planar imaging perpendicular to the entire tunnel [14], femoral aperture widening, graft-tunnel healing, and graft maturation inside the femoral tunnel were analyzed using the first reconstructed image slice placed perpendicular to the tunnel direction where complete circumferential tunnel walls were seen (Fig. 3). The intraarticular graft SNQ was measured using oblique sagittal proton density-weighted fat-suppressed (PD-FS) images.
Signal-to-noise quotient (SNQ)
The SNQ was used to evaluate the mechanical properties and ligamentization of the graft, as validated in previous studies [11, 15]. The signal intensity was measured on oblique sagittal PD-FS images in 0.05 cm2 circular regions of interest (ROI), tangent to the intraarticular ACL cross-section. The graft signal was measured intraarticularly using three equally spaced ROIs at the proximal, middle, and inferior thirds of the graft, and the average value was calculated. The proximal graft ROI was set adjacent to the femoral aperture. The distal graft ROI was set proximal to the tibial aperture, and its distal edge was aligned to the tibial joint surface. The midsubstance graft ROI was set such that its center was midway between the two previously placed ROIs at the level of the anterior and posterior intercondylar notches. The graft signal values were averaged as described by Weiler et al. [11] using a ROI placed at the base of the PCL, central to its broad tibial attachment. The background signal was measured 2 cm anterior to the patellar tendon. The mean signal intensity and standard deviations of each region were recorded based on image pixels as the absolute signal intensity, with a measurement accuracy of one decimal.
Afterward, the SNQ in each of the three graft regions was calculated to standardize the signal intensity using the following formula: SNQ = (graft signal—PCL signal) / background signal.
Femoral tunnel aperture widening
Femoral tunnel aperture widening was calculated by measuring the difference between the postoperative MR-derived tunnel diameter and the initial tunnel diameter drilled during surgery.
Graft-tunnel healing
According to Ge et al. [12], graft-tunnel healing was graded based on the signal intensity at the bone-graft interface as follows: (1) Complete healing with low signal intensity and no fluid at the bone-graft interface, (2) Partial healing with high signal intensity detected at a portion of the bone-tunnel interface, and (3) Poor healing with a high signal over the entire circumference of the bone-tunnel interface with poor attachment.
Graft maturity
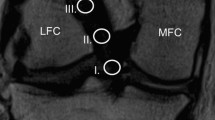
Graft maturity in the femoral tunnel was measured using a 4-grade system according to Howell et al. [13] as follows:
-
(I)
Homogeneous, low signal intensity like the PCL and patellar tendon.
-
(II)
Low signal intensity over at least 50% of the graft volume intermingled with portions with increased signal intensity.
-
(III)
High signal intensity over at least 50% of the graft volume intermingled with portions that had a normal ligament signal.
-
(IV)
Diffuse high signal intensity without normal ligament appearance.
The MRI scans were analyzed by two orthopedic surgeons (A.A. and H.H.), and all measurements were calculated twice with a 2-week interval and the mean was utilized. Each rater was blinded to the patient’s group and the score assigned by the other rater. The intraclass correlation coefficient (ICC) with a 95% confidence interval (CI) was calculated to assess interobserver reproducibility. The reliability of the mean (between raters 1 and 2) for the SNQ measurement, femoral aperture widening, graft- tunnel healing, and graft maturity was ICC 0.75 (95% CI 0.61–0.89), 0.70 (95% CI 0.58–0.82), 0.78 (95% CI 0.68–0.88), 0.79 (95% CI 0.71–0.87) respectively.
Statistical analysis
Data were inputted in the computer and analyzed using IBM SPSS Statistics 20.0 (Armonk, NY: IBM Corp). Categorical data were presented as numbers and percentages. Continuous data were tested for normality using the Shapiro–Wilk test. Quantitative data were expressed as range (minimum and maximum), mean, standard deviation, and median. Student’s t test was used to compare two groups for normally distributed quantitative variables, whereas the paired t test was used to compare two periods for normally distributed quantitative variables. The Mann–Whitney test was used to compare two groups for non-normally distributed quantitative variables, whereas the Wilcoxon signed-rank test was used to compare the abnormally distributed quantitative variables between two periods. The Friedman test was used to compare abnormally distributed quantitative variables between more than two periods or stages, This was a pilot study, and a priori power analysis was not performed; the Dunn's post-hoc test was used for pairwise comparisons. The significance level of the results was set at 5%.
Results
Subjective evaluation
The IKDC and Lysholm scores improved significantly in both groups (Table 2). Moreover, no significant differences in IKDC and Lysholm scores between the groups were detected at the final follow-up. Pre-injury Tegner score was higher in the PA-ACLR group than in the ST-ACLR group; this significant difference was maintained at the final follow up (Table 2). None of the patients had a ROM deficit at 24 months postoperatively. There was a significant difference in the VAS scores between the groups at 3- and 6-months point. Patients in the PA-ACLR group experienced more pain and tenderness at the harvest site early postoperatively. However, pain at the harvest site gradually improved over time, resulting in similar VAS scores between the two groups at 12 months postoperatively (Table 3).
Objective evaluation
Lachman test, pivot-shift grading, and ATT improved significantly in both groups. At final follow-up, none of the knees in either group showed significant laxity. There was no significant difference in the aforementioned parameters between the groups at the final follow-up (Table 4).
SNQ values
The mean SNQ was lower in the PA-ACLR group (3.9 ± 2.2) than in the ST-ACLR group (6.1 ± 4.7), but there was no significant difference (P = 0.143). Analysis of the values of the three graft segments (proximal, middle, distal) is presented in Table 5. There was a significant difference between the two groups only at the proximal third of the graft (P = 0.005), with the PA-ACLR group showing a consistently low value at the proximal third in most cases (Fig. 4), whereas no differences were identified in the middle or distal portion between both groups (P = 0.992, 0.625).
MRI scans obtained 12-month post ACL reconstruction of two matched patients, one from each group. a Standard reconstruction with intermediate signal intensity at the proximal part of the graft, poor healing at the femoral aperture (grade 3) and good graft maturity (grade 2). b Periosteal augmentation reconstruction with homogenous low signal intensity of the whole graft with good healing at femoral aperture (grade 1) and graft maturity grade 2
Graft healing, graft maturity, and femoral tunnel widening
Significant differences were observed between the two groups when graft healing, graft maturity, and femoral tunnel widening were compared. The PA-ACLR group showed better graft-tunnel healing and graft maturity, with significantly less femoral tunnel widening than the ST-ACLR group (Table 6).
Correlation between different variables
There was no correlation between the final subjective scores (Lysholm and IKDC) at 2 years postoperative and the MRI parameters obtained 1 year after surgery. However, a negative linear correlation was detected between the SNQ of the proximal part of the graft and Tegner activity scale in the PA-ACLR group (r = -0.581, p = 0.004).
Discussion
The most important finding of this study was that periosteal augmentation of the hamstring autograft, compared to standard ACL reconstruction, improved graft healing to the femoral tunnel walls, reduced femoral tunnel widening, and was associated with lower SNQ values of the intraarticular proximal part of the graft. These objective findings did not translate into significant clinical differences in patient-reported outcomes 2 years postoperatively. However, we observed a correlation between a lower SNQ score of the proximal part of the graft and a higher Tegner activity scale in the PA-ACLR group.
Graft healing to the bone tunnel has been a major concern that was extensively studied [4, 5, 16].
Experimental studies have demonstrated that periosteal tissue when placed in the graft-bone interface with the cambium layer facing the bony walls can induce bone and cartilage formation resulting in direct tendon to bone healing with a progressive increase in the interface failure strength [17,18,19]. Clinical studies enforced the use of the periosteum as a biological agent to enhance graft healing [6,7,8]. Chen et al. [8] reported mid-term results of arthroscopic transtibial single-bundle ACL reconstruction with periosteum-augmented hamstring tendon graft where two separate free periosteal flap were sutured to the hamstring tendon at the contact points with the femoral and tibial aperture. The authors reported excellent clinical scores, with good graft healing and minimal tunnel widening.
In the present study, a modification to the originally described periosteal augmentation technique was adopted, in which the periosteum was harvested, kept attached to the hamstring graft and implanted as one unit for better revascularization and graft incorporation after implantation. The periosteum was reflected so that the cambium layer faced the tunnel walls and covered the whole segment of the graft entering the femoral tunnel to maximize femoral graft integration; the tibial side of the graft was enhanced by preserving the tibial remnants of the native ACL.
There is growing evidence that femoral tunnel graft healing and integration are inferior in comparison to the tibial tunnel [20,21,22,23,24]. Putnis et al. [21] suggested that the femoral-side graft may be the rate-dependent area for healing of the entire graft and concluded that ACL graft rupture after 1 year is associated with MRI appearances of a high graft signal adjacent to and within the femoral tunnel aperture. In the current study, the periosteal flap was used in a way to maximize femoral graft integration and accelerate graft healing to the tunnel walls, thereby decreasing graft motion at the femoral aperture with a subsequent reduction in femoral tunnel widening.
MRI has proven to be a safe, noninvasive measure for evaluating ACL graft remodeling, mechanical properties, and tensile strength [25, 26]. The SNQ is used to assess graft edema and subsequently graft vascularity and biomechanical properties, as it is inversely proportional to the graft’s tensile strength [11, 27]. The mean SNQ of hamstring autograft 1 year after surgery was reported in various studies to be (1–22) [28,29,30]. Liu et al. [31] reported SNQ values of detached hamstring autograft (18.3 ± 7.7), compared to those of hamstring tendons, remained attached distally (12.6 ± 7.0). Cavaignac et al. [28] reported SNQ values of (5.9 ± 3.7) for quadrupled semitendinosus graft and (5.2 ± 4.5) for doubled semitendinosus and gracilis grafts. The mean SNQ in this study was relatively low in both study groups (ST-ACLR, 6.1 ± 4.7; PA-ACLR, 3.9 ± 2.8), which may be due to patient selection criteria, as we tried to control for variables that could affect graft healing such as meniscal deficiency, elevated PTS, high grade pivot, small graft size, and absence of tibial remnants [32,33,34], as well as adopting a slow rehabilitation program [35].
Higher SNQ values in the proximal part of the ACL graft (range, 12–23.2) compared to the intraarticular and tibial portions are consistent findings reported in MRI-based graft healing studies [31, 36]. In our study, SNQ of the proximal portion of the graft was consistently lower in the PA-R group (2.9 ± 3.3) than in the ST-R group (9.6 ± 9.2) and even lower than values reported in the literature [31, 37] this may lead to probably better graft rupture rate based on findings in previous studies [21]. The SNQ values of the middle and distal portions of the graft were consistently low in both study groups, which may be attributable to the preservation of the tibial remnant of the native ACL, as these values are consistent with those reported in another study investigating the effect of tibial remnant preservation [38].
In the present study, the tunnel aperture was selected for analysis to evaluate tunnel widening and graft-tunnel healing because of existing histological evidence that it is an important site for graft incorporation [16, 24]. Previous studies have demonstrated inferior graft healing to the femoral tunnel walls in comparison to the tibial tunnel, with complete healing ranging from (21–26%) [39, 40]. In the present study, graft-tunnel healing at the aperture was considered complete (grade 1) in 65% of patients in the PA-ACLR group compared to 28% of patients in the ST-ACLR group, which contributes to existing evidence that the periosteal flap could enhance the graft-bone interface and promote healing through direct and indirect integration, as discussed previously.
Significant tunnel widening with the use of hamstring tendon autografts has been previously reported [24, 41]. In the present study, significant reduction in femoral tunnel widening percentage could be detected in the PA-ACLR group (2.3% ± 9.9%) compared to the ST-ACLR group (30.3% ± 18.3%). Moreover, tunnel narrowing was detected in few cases in the PA-ACLR group (24%), and this may be attributed to the osteogenic properties of the periosteal tissue, these values are comparable to those of the bone patellar tendon bone (BTB) graft [42, 43]. Robert and Es-sayeh [44] studied the effect of the periosteum in preventing femoral tunnel widening and found that there was a significant reduction in femoral tunnel enlargement at the tunnel outlet with the use of a periosteal flap compared to standard reconstruction, although tunnel widening was constant in both study groups.
The clinical significance of the MRI-based graft appearance, tunnel widening, and graft tunnel integration remains unclear [45, 46]. In the current study, there was no significant correlation between the MRI parameters studied and clinical scores (IKDC, Lysholm) at 2 years postoperative, with both groups having good-to-excellent IKDC and Lysholm mean scores, as well as nearly normal ATT, indicating that patients in both cohorts had little to no limitation with daily or sporting activities and minimal or zero symptoms postoperatively. The VAS scores at 3- and 6-month postoperatively differed significantly between both groups. Patients in the PA-ACLR group experienced more pain at the harvest site, which was expected due to periosteum stripping at the anteromedial tibial border; however, the patients experienced gradual improvements. At 2 years post-operatively, both groups experienced no pain.
There was a weak negative linear correlation between the proximal intraarticular graft signal and Tegner scale, with the correlation reaching significance only in the PA-ACLR group (moderate correlation, r = −0.581, p = 0.004). The median postoperative Tegner score in the ST-ACLR group was 6 (pre-injury score: 6, range: 5–8) and 7 (pre-injury score: 7, range: 5–8) in the PA-ACLR group, indicating that patients in both groups returned to near their pre-injury levels of activity. Despite having a higher pre-injury Tegner activity score, a large percentage of patients in the PA-ACLR group returned to their high pre-injury level with no failures at 2-year follow up; these findings are consistent with those of previous studies correlating the high SNQ of the femoral portion of the graft with inferior outcomes [21, 30, 46].
This study had several limitations. First, due to the lack of relevant data, we were not able to calculate the appropriate sample size in advance and the small number of participants might have resulted in a relatively low statistical power. Second, graft SNQ is influenced by several variables, including knee position at the time of the scan and technical issues such as sequence and scanner characteristics, reconstruction algorithms, gray scale displays, surgical technique, and graft bending angle [30]. These differences raise doubts about the use of MRI signal intensity measurements as a quantitative method for measuring graft maturation. Third, the heterogeneity of methods for measuring signal intensities and MRI slice selection for measuring tunnel widening and graft healing prevented a direct comparison between studies of similar interest. Four, MRI was performed at least 1 year after surgery in all patients, although studies have shown that graft maturation and normalization of MRI signals can progress up to 2 years postoperatively [47]. However, despite the continuation of ligamentization, it is important to evaluate the SNQ at 1-year post-surgery, a time point at which most patients want to return to sports. Fifth, anterior tibial translation measured on a standing single leg stance lateral radiograph is greatly influenced by the PTS and it may not be suitable for patients’ comparison, however, this influence is minimized since we included only patients with PTS less than 12 degrees.
Conclusion
Periosteal wrapping of hamstring tendon autografts is associated with improved graft-tunnel healing and maturation, as well as a lower incidence of femoral tunnel widening based on MRI analysis 1-year after ACL reconstruction. However, the patient-reported outcomes and measured laxity were similar between the two study groups at 2 years postoperative evaluation.
Data availability
Data are available on request to authors.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- PCL:
-
Posterior cruciate ligament
- LCL:
-
Lateral collateral ligament
- ST-ACLR:
-
Standard anterior cruciate ligament reconstruction
- PA-ACLR:
-
Periosteal augmented anterior cruciate ligament reconstruction
- ATT:
-
Anterior tibial translation
- ROI:
-
Region of interest
- IKDC:
-
International knee documentation score
- SNQ:
-
Signal to noise quotient
- MRI:
-
Magnetic resonance imaging
- VAS:
-
Visual analogue scale
- BTB:
-
Bone patellar tendon bone graft
References
Baawa-Ameyaw J, Plastow R, Begum FA, Kayani B, Jeddy H, Haddad F (2021) Current concepts in graft selection for anterior cruciate ligament reconstruction. EFORT Open Rev 6:808–815
Sollberger VD, Korthaus A, Barg A, Pagenstert G (2022) Long-term results after anterior cruciate ligament reconstruction using patellar tendon versus hamstring tendon autograft with a minimum follow-up of 10 years—a systematic review. Arch Orthop Trauma Surg 143:4277–4289
Amano H, Tanaka Y, Kita K, Uchida R, Tachibana Y, Yonetani Y (2019) Significant anterior enlargement of femoral tunnel aperture after hamstring ACL reconstruction, compared to bone—patellar tendon—bone graft. Knee Surg Sports Traumatol Arthrosc 27:461–470
Petersen W, Laprell H (2000) Insertion of autologous tendon grafts to the bone: A histological and immunohistochemical study of hamstring and patellar tendon grafts. Knee Surg Sports Traumatol Arthrosc 8:26–31
Ekdahl M, Wang JH, Ronga M, Fu FH (2008) Graft healing in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 16(10):935–947
Kuang GM, Yau WP, Lu WW, Chiu KY (2010) Osteointegration of soft tissue grafts within the bone tunnels in anterior cruciate ligament reconstruction can be enhanced. Knee Surg Sports Traumatol Arthrosc 18(8):1038–1051
Chen CH, Chen WJ, Shih CH, Chou SW (2004) Arthroscopic anterior cruciate ligament reconstruction with periosteum-enveloping hamstring tendon graft. Knee Surg Sports Traumatol Arthrosc 12(5):398–405
Chen C, Chang C, Su C, Wang K, Liu H, Yu C, Wong C, Wang I (2010) Arthroscopic single-bundle anterior cruciate ligament reconstruction with periosteum-enveloping hamstring tendon graft: clinical outcome at 2 to 7 years. Arthroscopy 26(7):907–917
Youn I, Jones DG, Andrews PJ, Cook MP, Suh JKF (2004) Periosteal augmentation of a tendon graft improves tendon healing in the bone tunnel. Clin Orthop Relat Res 419:223–231
Ma Y, Murawski CD, Rahnemai-Azar AA, Maldjian C, Lynch AD, Fu FH (2015) Graft maturity of the reconstructed anterior cruciate ligament 6 months postoperatively: a magnetic resonance imaging evaluation of quadriceps tendon with bone block and hamstring tendon autografts. Knee Surg Sports Traumatol Arthrosc 23:661–668
Weiler A, Peters G, Mäurer J, Unterhauser FN, Südkamp NP (2001) Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging: a two-year study in sheep. Am J Sports Med 29:751–761
Ge Y, Li H, Tao H, Hua Y, Chen J, Chen S (2015) Comparison of tendon–bone healing between autografts and allografts after anterior cruciate ligament reconstruction using magnetic resonance imaging. Knee Surg Sports Traumatol Arthrosc 23:954–960
Howell SM, Clark JA, Farley TE (1992) Serial magnetic resonance study assessing the effects of impingement on the MR image of the patellar tendon graft. Arthroscopy 8:350–358
Fules PJ, Madhav RT, Goddard RK, Newman-Sanders A, Mowbray MAS (2003) Evaluation of tibial bone tunnel enlargement using MRI scan cross-sectional area measurement after autologous hamstring tendon ACL replacement. Knee 10:87–91
Gohil S, Annear PO, Breidahl W (2007) Anterior cruciate ligament reconstruction using autologous double hamstrings: a comparison of standard versus minimal debridement techniques using MRI to assess revascularization–a randomised prospective study with a one-year follow-up. J Bone Joint Surg 89-B:1165–1171
Lu H, Chen C, Xie S, Tang Y, Qu J (2019) Tendon healing in bone tunnel after human anterior cruciate ligament reconstruction: a systematic review of histological results. Journal of Knee Surgery 32:454–462
Roberts SJ, van Gastel N, Carmeliet G, Luyten FP (2015) Uncovering the periosteum for skeletal regeneration: the stem cell that lies beneath. Bone 70:10–18
Chen CH, Chen WJ, Shih CH, Yang CY, Liu SJ, Lin PY (2003) Enveloping the tendon graft with periosteum to enhance tendon-bone healing in a bone tunnel: a biomechanical and histologic study in rabbits. Arthroscopy 19:290–296
Kyung HS, Kim SY, Oh CW, Kim SJ (2003) Tendon-to-bone tunnel healing in a rabbit model: The effect of periosteum augmentation at the tendon-to-bone interface. Knee Surg Sports Traumatol Arthrosc 11:9–15
Panos JA, Webster KE, Hewett TE (2020) Anterior cruciate ligament grafts display differential maturation patterns on magnetic resonance imaging following reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc 28:2124–2138
Putnis SE, Oshima T, Klasan A, Grasso S, Neri T, Fritsch BA, Parker DA (2021) Magnetic resonance imaging 1 year after hamstring autograft anterior cruciate ligament reconstruction can identify those at higher risk of graft failure: an analysis of 250 cases. Am J Sports Med 49:1270–1278
Mayr R, Smekal V, Koidl C, Coppola C, Fritz J, Rudisch A, Kranewitter C, Attal R (2017) The knee tunnel widening after ACL reconstruction with aperture screw fi xation or all-inside reconstruction with suspensory cortical button fixation. Volumetric measurements on CT and MRI scans. Knee 24(5):1047–1054
Zhu J, Marshall B, Tang X, Linde MA, Fu FH, Smolinski P (2022) ACL graft with extra-cortical fixation rotates around the femoral tunnel aperture during knee flexion. Knee Surg Sports Traumatol Arthrosc 30:116–123
Yue L, Defroda SF, Sullivan K, Garcia D, Owens BD (2020) Mechanisms of bone tunnel enlargement following anterior cruciate ligament reconstruction. J Bone Joint Surg Rev 8:1–9
Biercevicz AM, Akelman MR, Fadale PD, Hulstyn MJ, Shalvoy RM, Badger GJ, Tung GA, Oksendahl HL, Fleming BC (2015) MRI volume and signal intensity of ACL graft predict clinical, functional, and patient-oriented outcome measures after ACL reconstruction. Am J Sports Med 43:693–699
DeFrodaODonnell SFRM, Fadale PD, Owens BD, Fleming BC (2021) The role of magnetic resonance imaging in evaluating postoperative ACL reconstruction healing and graft mechanical properties: a new criterion for return to play? Phys Sportsmed 49:123–129
Malahias MA, Capece FM, Ballarati C, Viganò M, Marano M, Hofbauer M, Togninalli D, de Girolamo L, Denti M (2022) Sufficient MRI graft structural integrity at 9 months after anterior cruciate ligament reconstruction with hamstring tendon autograft. Knee Surg Sports Traumatol Arthrosc 30:1893–1900
Cavaignac E, Marot V, Faruch M, Reina N, Murgier J, Accadbled F, Berard E, Chiron P (2018) Hamstring graft incorporation according to the length of the graft inside tunnels. Am J Sports Med 46:348–356
Li Q, Zhang Y, Zhan L, Han Q, Wu M, Zhang N (2019) Correlation analysis of magnetic resonance imaging-based graft maturity and outcomes after anterior cruciate ligament reconstruction using international knee documentation committee score. Am J Phys Med Rehabil 98:387–391
Yau WP, Chan YC (2023) Evaluation of graft Ligamentization by MRI after anterior cruciate ligament reconstruction. Am J Sports Med 51:1466–1479
Liu S, Li H, Tao H, Sun Y, Chen S, Chen J (2018) A randomized clinical trial to evaluate attached hamstring anterior cruciate ligament graft maturity with magnetic resonance imaging. Am J Sports Med 46:1143–1149
Okutan AE, Kalkışım M, Gürün E, Ayas MS, Aynacı O (2022) Tibial slope, remnant preservation, and graft size are the most important factors affecting graft healing after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 30(5):1584–1593
Huang H, Nagao M, Nishio H, Kaneko H, Saita Y, Takazawa Y, Ikeda H, Kaneko K, Ishijima M (2021) Remnant preservation provides good clinical outcomes after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 29:3763–3772
Vari N, Marot V, Cavaignac M, Vieira TD, Bérard É, Cavaignac E (2023) Factors affecting graft remodeling and anterior cruciate ligament reconstruction: MRI study of 180 knees. Am J Sports Med 51(8):2073–2078
Tajima T, Yamaguchi N, Nagasawa M, Morita Y, Nakamura Y, Chosa E (2019) Early weight-bearing after anterior cruciate ligament reconstruction with hamstring grafts induce femoral bone tunnel enlargement : a prospective clinical and radiographic study. BMC Musculoskelet Disord 20(1):274. https://doi.org/10.1186/s12891-019-2653-6
Hofbauer M, Soldati F, Szomolanyi P, Trattnig S, Bartolucci F, Fu F, Denti M (2019) Hamstring tendon autografts do not show complete graft maturity 6 months postoperatively after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 27:130–136
Ahn JH, Jeong HJ, Lee YS, Park JH, Lee JH, Ko TS (2016) Graft bending angle is correlated with femoral intraosseous graft signal intensity in anterior cruciate ligament reconstruction using the outside-in technique. Knee 23:666–673
Shimodaira H, Tensho K, Koyama S, Iwaasa T, Kumaki D, Yoshida K, Horiuchi H, Takahashi J (2023) Effect of a new remnant-preserving technique with anatomical double-bundle anterior cruciate ligament reconstruction on MRI-based graft maturity: a comparison cohort study. Knee Surg Sports Traumatol Arthrosc 31:2394–2405
Putnis S, Neri T, Grasso S, Linklater J, Fritsch B, Parker D (2020) ACL hamstring grafts fixed using adjustable cortical suspension in both the femur and tibia demonstrate healing and integration on MRI at one year. Knee Surg Sports Traumatol Arthrosc 28:906–914
Putnis SE, Oshima T, Klasan A, Grasso S, Fritsch BA, Coolican MRJ, Parker DA (2021) Adjustable suspension versus hybrid fixation in hamstring autograft anterior cruciate ligament reconstruction. Knee 28:1–8
Bhullar R, Habib A, Zhang K, de Sa D, Horner NS, Duong A, Simunovic N, Espregueira-Mendes J, Ayeni OR (2019) Tunnel osteolysis post-ACL reconstruction: a systematic review examining select diagnostic modalities, treatment options and rehabilitation protocols. Knee Surg Sports Traumatol Arthrosc 27:524–533
Clatworthy MG, Annear P, Bulow JU, Bartlett RJ (1999) Tunnel widening in anterior cruciate ligament reconstruction: a prospective evaluation of hamstring and patella tendon grafts. Knee Surg Sports Traumatol Arthrosc 7:138–145
DeFroda SF, Karamchedu NP, Owens BD, Bokshan SL, Sullivan K, Fadale PD, Hulstyn MJ, Shalvoy RM, Badger GJ, Fleming BC (2018) Tibial tunnel widening following anterior cruciate ligament reconstruction: a retrospective seven-year study evaluating the effects of initial graft tensioning and graft selection. Knee 25:1107–1114
Robert H, Es-sayeh J (2004) The role of periosteal flap in the prevention of femoral widening in anterior cruciate ligament reconstruction using hamstring tendons. Knee Surg Sports Traumatol Arthrosc 12(1):30–35
Li H, Chen J, Li H, Wu Z, Chen S (2017) MRI-based ACL graft maturity does not predict clinical and functional outcomes during the first year after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 25:3171–3178
Li HY, Li H, Wu ZY, Chen JW, Chen SY (2018) MRI-based tendon bone healing is related to the clinical functional scores at the first year after anterior cruciate ligament reconstruction with hamstring tendon autograft. Knee Surg Sports Traumatol Arthrosc 26:615–621
Panos JA, Devitt BM, Feller JA, Klemm HJ, Hewett TE, Webster KE (2021) Effect of time on MRI appearance of graft after ACL reconstruction: a comparison of autologous hamstring and quadriceps tendon grafts. Orthop J Sports Med 9(9):23259671211023510
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Ahmed Helal: Writing, statistical analysis, performing surgeries. Osama El-Gebaly: Patient recruitment, clinical examination, and radiological measurements. Hany Hamed: Patient recruitment, clinical examination, and radiological measurements. Ali M. Omran: Patient recruitment, clinical examination, and radiological measurements. Elsayed Elforse: Performing surgeries, supervision, and coordinator.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Approval was obtained from the ethics committee of Tanta faculty of medicine. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent
All patients submitted their written informed consent to participate in this investigation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Helal, A., El-Gebaly, O., Hamed, H. et al. Periosteal wrapping of the hamstring tendon autograft improves graft healing and prevents tunnel widening after anterior cruciate ligament anatomic reconstruction. Arch Orthop Trauma Surg 144, 2711–2722 (2024). https://doi.org/10.1007/s00402-024-05356-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-024-05356-9