Abstract
Introduction
One of the main causes of RSA failure is attributable to the malpositioning of the glenoid component. Initial experiences with computer-assisted surgery have shown promising results in increasing the accuracy and repeatability of placement of the glenoid component and screws. The aim of this study was to evaluate the functional clinical results, in terms of joint mobility and pain, by correlating them with intraoperative data regarding the positioning of the glenoid component. The hypothesis was that the lateralization more than 25 mm of the glenosphere can led to better stability of the prosthesis but should pay in term of a reduced range of movement and increased pain.
Materials and methods
50 patients were enrolled between October 2018 and May 2022; they underwent RSA implantation assisted by GPS navigation system. Active ROM, ASES score and VAS pain scale were recorded before surgery. Preoperative data about glenoid inclination and version were collected by pre-op X-Rays an CT. Intraoperative data—inclination, version, medialization and lateralization of the glenoid component—were recorded using computer-assisted surgery. 46 patients had been further clinically and radiographically re-evaluated at 3-months, 6-months, 1-year, and 2-years follow-up.
Results
We found a statistically significant correlation between anteposition and glenosphere lateralization value (DM − 6.057 mm; p = 0.043). Furthermore a statistically significant correlation has been shown between abduction movement and the lateralization value (DM − 7.723 mm; p = 0.015). No other statistically significant associations were found when comparing the values of glenoid inclination and version with the range of motion achieved by the patients after reverse shoulder arthroplasty.
Conclusion
We observed that the patients with the best anteposition and abduction results had a glenosphere lateralization between 18 and 22 mm. When increasing the lateralization above 22 mm or reducing it below 18 mm, on the other hand, both movements considered decreased their range.
Level of evidence
Level IV; Case Series; Treatment Study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Every year approximately 1% of implanted shoulder prosthesis results in aseptic loosening and surgical revision [1]. One of the main causes of this failure is attributable to the malpositioning of the glenoid component [2]. Postoperative instability is the most common complication reported from 2.4 to 31% in the current literature [3]. Implantation of a reverse shoulder arthroplasty (RSA) with superior inclination of the baseplate is associated with an increased rate of complications [4,5,6].
Superior inclination of the baseplate increases the stresses at the implant–bone interface leading to impingement between the inferior humeral polyethylene insert and scapular pillar, causing medial polyethylene wear, scapular notching and eventual glenoid implant loosening [4,5,6]. Furthermore, superior inclination of the baseplate has been shown to be associated with decreased shoulder range of motion [7, 8].
The optimal position of the reverse baseplate is flush to the inferior rim of the glenoid surface without superior inclination, in an attempt to optimize impingement-free range of motion avoiding scapular notching and glenoid loosening [9, 10].
A medialization of the center of rotation leads to less micro motions at the implant/bone surface and therefore to a greater survival of the prosthesis itself; however, excessive medialization leads to greater instability and reduced range of motion [11]. On the contrary, the lateralization of the center of rotation increases implant stability, but also increasing the shear forces at the implant/bone interface, thus leading to a lower survival of the implant. The lower inclination of the glenosphere can compensate for an insufficient lateralization avoiding the instability of the implant allowing a greater distalization of the humerus and increasing the deltoid tension of the prosthesis [12].
For all these reasons, an accurate and precise glenoid positioning correlates with optimal function and longevity of implants [9].
The lack of static and reliable landmarks on the shoulder, the scarcity of the bone stock often altered in the course of an arthritis and the limited access to the scapula through any of the available shoulder approaches can complicate the process of the glenoid positioning.
Initial experience with computer-assisted surgery [13], has shown promising results in increasing the accuracy and repeatability of the placement of the glenoid component, in particular the position and orientation of the glenoid component and the screws. It is not yet known whether the accomplishment of improved positioning techniques translates into better clinical outcomes and the literature regarding computer-assisted shoulder surgery [15, 16] is still rather poor.
The aim of this study is to evaluate the functional and clinical results, in terms of joint mobility and pain, by correlating them with intraoperative data regarding the positioning of the glenoid component. The hypothesis is that the lateralization more than 25 mm of the glenosphere can led to the better stability of the prosthesis but could pay in terms of reduced range of movement and increased pain.
Materials and methods
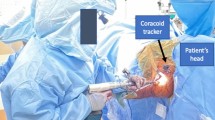
All patients treated with reverse shoulder arthroplasty, where the Guided Personalized Surgery (GPS) navigation system (Exactech, Gainsville, FL, USA) was used to assist the implantation of the Equinoxe reverse shoulder prosthesis (Exactech, Naples, FL, USA), were included in this study.
All the included subjects had been treated at our institute from October 2018 to May 2022.
A total of 50 patients were considered of which 16 males and 34 females. The mean age at the time of surgery was 73.6 years (ranging between 51 and 87 years).
Indications for surgery were: 30 glenohumeral arthrosis, 20 rotator cuff rupture arthropathies, 27 of these were right sided cases and 23 were left sided cases.
Active Range of Motion (ROM) with evaluation of external rotation, internal rotation, abduction and anteposition, American Shoulder and Elbow Surgeons Score (ASES score), and Visual Analogue Scale (VAS) pain scale were analysed before surgery.
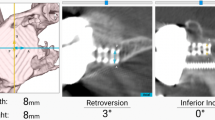
X-rays and CT scans were carried out to gain data of preoperative glenoid version and inclination measured through Orthoblue software. In addition, the glenoid component inclination, version, seating and medialisation were planned using the Orthoblue software and recorded.
All surgical procedures were performed by the same senior surgeon (L.T.), who had already executed more than 50 Equinoxe RSA using navigation system before 2018 [17].
The data were collected intraoperatively—using computer-assisted surgery—about inclination, version, medialization and lateralization of the glenoid component (thickness of the glenoid component from which the absolute value of medialization was subtracted, see Fig. 1).
Graphic image of how the lateralization of the COR was calculated, as well as the difference between the medialization and the length of the glenoid component. l = length glenoid component (36 mm diameter glenosphere: l = 22 mm, 38 mm diameter glenosphere: l = 23 mm, 42 mm diameter glenosphere: l = 25 mm). M medialization. L lateralization (calculated as l–M)
The type of baseplate used, the presence of any intraoperative complications and associated further surgical procedures were registered as well.
We re-evaluated all patients by objective examination, ASES score, VAS pain scale (1–10 score) and radiographic analysis at 3 months, 6 months, 1 year and then annually.
We gathered the 3 months, 6 months, 1-year follow-up for the whole study population. 2-year follow-up information were collected for 46 patients (min 24.1–max 30.5 months); we could not complete the follow-up for 4 patients.
Statistical analysis
All statistical analyses were performed using R software version 4.1.1.
Numerical variables were described through mean and standard deviation or median and interquartile range (IQR), whereas categorical variables were reported in terms of absolute frequencies and percentages. The degree of association between the outcomes of interest and the numerical and categorical variables of interest was assessed through the estimation of simple linear regression models. The relationships between external rotation, internal rotation, abduction, anteposition, and preoperative and planning measures were investigated analogously, with a view to analyse possible mediating effects by active ROM measurements. The results obtained were reported in terms of the difference between the means (DM), with corresponding 95% confidence intervals.
Results
The mean preoperative glenoid version was − 6.5° ± 6.1° (min − 20° and max 6°), the mean preoperative inclination was 1.7° ± 6.3° (min − 11° and max 19°). The planned mean glenoid version was − 2.1° ± 3.0° (min − 9° and max 3°), the planned mean inclination was − 1.8° ± 2.3° (min − 8° and max 0°).
From the preoperative to the intraoperative phase, we obtained a mean version correction of − 4.4° ± 5.9° (min − 20° and max 7°) and a mean inclination correction of 3.5 ± 6.3° (min − 11° and max 20°) (Table 1).
Regarding the difference between planned and intraoperative measurements, the inclination of the glenoid component was increased by 1° in five patients whereas in the remaining 45 cases the prosthetic device was implanted according to plan.
The planned mean medialization was − 3.6 mm ± 1.8 mm (min 0 mm and max − 7 mm), which corresponded to the reaming performed intraoperatively for all 50 implanted prostheses.
The mean lateralization obtained of 19.4 mm ± 1.8 mm (min 16 mm and max 23 mm) followed from this figure.
Of the 46 patients evaluated at 2-year follow-up, 26 had a lateralization between 18 and 22 mm, while 20 had lateralisation values > 22 mm or < 18 mm. Out of 26 patients, 24 had abduction and anteposition values ≥ 90° (92.3%) while 2 patients reached < 90° (7.7%), but had co-morbidities. Of the 20 patients outside the range: 10 had abduction and anteposition > 90° (50%) and a further 10 had abduction and anteposition < 90°, with absence of comorbidities (50%).
The value of the planned seating was 94.3% ± 6.1% (min 74% and max 100%). The baseplate used were: 23 Standard, 15 Augment 8° posterior, 8 Augment 10° superior.
The intraoperative adverse events registered were: two coracoid fractures and one GPS failure, which prevented the use of navigation during implantation of the glenoid component.
The months following arthroplasty implantation involved two traumatic dislocations of the prosthetic implant, causing both patients to undergo prosthetic revision; two prosthetic infections, both within 3 months from the operation, requiring surgical cleaning and replacement of the mobile components (Fig. 2). One of the two cases of the infection required a further revision with the removal of the infected prosthesis and replacement with an antibiotic-loaded spacer due to the recurrence of signs and symptoms of infection.
At the preoperative clinical evaluation, the patients presented a mean anteposition of 88.9° ± 28.3° (min 30° and max 160°), a mean abduction of 82.6° ± 33.9° (min 30° and max 160°), a mean external rotation of 22° ± 18.2° (min 0° and max 45°) and a mean internal rotation of 2.7 ± 2.2 (min 0 and max 7). Numerical values corresponding to each anatomical level were assigned to evaluate the range of internal rotation: gluteus = 0, sacrum = 1, L5 = 2, L4 = 3, L3 = 4, L2 = 5, L1 = 6, T12 = 7, T11 = 8, T10 = 9, T9 = 10, T8 = 11, T7 = 12. The mean ASES score was 42.3 ± 17.5 (min 6 and max 81.34) and the mean VAS was 6.2 ± 1.8 (min 1 and max 10).
At the 3-months follow-up, all patients achieved a mean ROM with an anteposition of 101.2° ± 26.7° (min 40° and max 160°), an abduction of 92.3° ± 21.6° (min 45° and max 160°), an external rotation of 22.5° ± 15.9° (min 0° and max 60°) and an internal rotation of 2.2 ± 2.5 (min 0 and max 9). The mean ASES score was of 68.35 ± 14.79 (min 26.9 and max 98.14) and the mean VAS pain score was 2.1 ± 2.4 (min 0 and max 8).
At the 6-months follow-up, patients presented a mean ROM with an anteposition of 119.1° ± 32.3° (min 60° and max 180°), an abduction of 110.1° ± 33.4° (min 60° and max 180°), an external rotation of 36.9° ± 18.2° (min 10° and max 70°) and an internal rotation of 3.7 ± 3.1 (min 0 and max 9). The mean ASES score was 78.09 ± 12.99 (min 24.96 and max 96.6) and the mean VAS pain score was 1.7 ± 2.1 (min 0 and max 8).
At the 1-year follow-up, patients achieved a mean ROM with an anteposition of 130.2° ± 33.2° (min 70° and max 180°), an abduction of 121.2° ± 36.4° (min 70° and max 180°), an external rotation of 40.9° ± 21.9° (min 10° and max 70°) and an internal rotation of 3.7 ± 2 (min 0 and max 12). The mean ASES score was 83 ± 11.5 (min 56.6 and max 98.14) and the mean pain score was 0.9 ± 1.4 (min 0 and max 4).
At the 2-years follow-up, patients presented a mean ROM with an anteposition of 136.8° ± 39.6° (min 70° and max 180°), an abduction of 123.6° ± 44.1° (min 70° and max 180°), an external rotation of 44.8° ± 24.1° (min 15° and max 90°) and an internal rotation of 5.3 ± 4.0 (min 0 and max 12). The mean ASES score was 82.72 ± 18.7 (min 55 and max 98.14) and the mean pain score was 0.8 ± 1.7 (min 0 and max 7).
External rotation showed a twofold increase in mean amplitude from 22° preoperatively to 44.8° at the 2-years follow-up, with 69.6% of patients showing a ROM of 30° or more at follow-up.
Internal rotation is the movement that showed the least increase as compared to the mean preoperative level, moving from L5 to L2 on average. Only 13 patients out of a total of 46 reached or exceeded the T12 level more than one year after surgery.
Two statistically significant correlations were also shown between the amplitude of anteposition and the medialization and lateralization values of the prosthetic implant (p = 0.043), and again between the amplitude of abduction and the medialisation and lateralisation values (p = 0.015).
No other statistically significant associations were found when comparing the values of postoperative glenoid inclination and version with the range of motion achieved by the patients after reverse shoulder arthroplasty.
No statistically significant associations were found in regards to pain values between planned glenoid version (p = 0.600), planned glenoid inclination (p = 0.344), medialization (p = 0.441) and lateralization (p = 0.441).
Discussion
The literature concerning intraoperative navigation in shoulder prosthetics is currently not very extensive. Studies attempted to establish the usefulness of three-dimensional surgical planning [18, 19] and the importance of intraoperative feedback on the change in version and glenoid inclination.
Navigation has been shown to improve the accuracy of the positioning of the glenoid component [1, 16] and screw fixation [14] even with more experienced surgeons [20].
So far, however, the accuracy of glenoid positioning has not yet been correlated with better clinical results as compared to standard techniques [1, 15, 16].
There are three main aspects of glenoid positioning that have to be considered when implanting the prosthetic device [20]: seating, version and tilt. The ideal seating value should be greater or equal to 90% of the implant’s surface area [20], its version between 5° anteversion and 15° retroversion (although most researchers use 0° to − 10° as the range) and the implant’s lower inclination less than 20° [21].
In our study, we followed the values suggested in literature, however, we did not find any statistically significant correlation between the inclination and version values and the postoperative clinical results. Similarly to these studies, we observed an increased use of baseplate implants with wedge [19, 20]: exactly half of the implants we implanted had superior or posterior wedge.
Our findings (as highlighted in Table 2) suggest the presence of a statistically significant correlation between anteposition (assessed at last clinical follow-up) and the medialization and lateralization value (DM − 6.057 mm; p = 0.043). Furthermore, there is also a statistically significant correlation between the abduction movement (assessed at last clinical follow-up) and the medialization and lateralization value (DM − 7.723 mm; p = 0.015).
As far as we know, the findings highlighted in this research is not reported by any previous study using intraoperative navigation.
Our results show that a greater glenoid component lateralization (> 22 mm) leads to a decrease in ROM in abduction and anteposition, whereas there is no correlation with external rotation. This finding is in contrast with the current literature consisting of studies that use specific software to evaluate the ROM after virtual Reverse Total Shoulder Arthroplasty (RTSA) surgery. Werner et al. [22] showed that by increasing the mean lateralisation of the Centre of Rotation (COR) to 2.5 mm (increasing the size of the glenosphere from 36 to 38 mm), both the anteposition and external rotation movements improved. In the study by Kim et al. [23] patients treated with a lateralizing implant had significantly greater adduction, abduction and external rotation. Keener et al. [24]. achieved a significantly greater adduction, abduction and external rotation with a progressively greater lateralisation, while internal rotation was better at 10 mm lateralisation.
Also Berhouet et al. [25] performed a cadaver study which showed that prostheses with a 7 mm or 10 mm lateralized COR and a 42 mm glenosphere had an increased elevation.
A population study of 146 patients was also carried out by Rhee et al. [26]. who showed how patients with greater medialization exhibited an improved pain score at the expense of reduced external rotation.
In a recent radiographic study (Chul-Hyun Cho, Du-Han Kim, Hyeong-Uk Choi, Byung-Chan Choi, Ji-Hoon Kim, unpublished data) there was one finding in line with our result. The postoperative acromio-humeral interval showed an association with active range of motion in patients who had undergone RTSA. In particular, excessive distalization reduced anteposition and external rotation.
The patients whose COR lateralization ranges between 18 and 22 mm, who completed all the outpatient follow-ups, reached a mean anteposition amplitude of 138.7° and a mean abduction amplitude of 120.7°. Only two patients did not reach an amplitude of movement equal to or greater than 90° for both of the above-mentioned movements. This result is likely due to the copathologies of the two patients: rheumatoid arthritis and Parkinson’s disease [27]. Only one patient achieved an angle corresponding to 90° for both movements, but in her post-operative course she suffered a traumatic dislocation of the prosthetic implant. All the remaining patients achieved greater ranges of motion of up to 180°. These data could be explained by an “ideal” deltoid length range in which the muscle performs better.
This group showed also better clinical scores, maybe driven by increased anteposition and abduction.
The mean ASES score among all patients falling within the lateralization range we considered is 85.32%, higher than the mean calculated among all patients at 2-years follow-up (Fig. 3).
The ASES score exceeds 80 points in 26 patients (56.5%) at last FU, while only 5 patients had a score < 80 points. The latter experienced a decline in function of the prosthetic shoulder, greater than 20 points, between the first and second year after surgery. The cause for this trend could be due to rheumatoid arthritis for two patients, Parkinson's disease for one patient, and age > 80 years for the remaining 2.
As far as the VAS scale is concerned, the mean value decreased from 6.2 to 2.1 in the first 3 months after surgery and then to < 1 after one year (Fig. 4). At the last outpatient appointment, 33 out of 46 patients, i.e. 71.7% of our sample, perceived no pain.
5 patients, on the other hand, complained of moderate-to-severe pain, four of which appeared on the occasion of complications such as infection and prosthetic dislocation, while the remaining one complained a worsening of rheumatoid arthritis.
There are several limitations to the use of computer-assisted surgery. The use of navigation exposes to the risk of coracoid fracture due to the positioning of the tracker, which occurred on two occasions in our study (4%).
In our study, there was one case of malfunction of the navigation system (2%) in line with what has been described in other studies in literature [17, 20], resulting in manual implantation of the glenoid component.
The main limitation of the study is the lack of postoperative imagines analysis to confirm prosthesis placement according to the preoperative planning. Several studies have been published about navigation accuracy and precision.
Jones et al. [28] evaluated accuracy and precision in glenoid positioning with a cadaveric study comparing preoperative plan and postoperative TC. They reported an average error from the preoperative plan of 1.9 ± 1.9° for version and 2.4 ± 2.4° for inclination of glenoid component. Larose et al. [29] compared preoperative planning and intraoperative navigated glenoid position in a large study population. They reported minimal deviation in the intraoperative execution of the preoperative plan with respect to version (0.6° ± 1.96°), inclination (0.2° ± 2.04°) and starting point on the glenoid face (1.90 mm ± 1.2 mm). Nguyen et al. [30] conduced an in-vitro randomized controlled trial with cadaveric specimen performing a postoperative TC. The mean absolute error (from the target value of 0°) in glenoid version was 1.5 ± 1.9° for the computer assisted method.
When considering these results, we assumed the navigation as precise and accurate process for glenoid placement, consequently we did not conduced postoperative imaging analysis to compare component position with preoperative planning.
The main limitation of this technology is that the humeral osteotomy, and therefore the offset and global distalization given by the positioning of the implant, was completely neglected by the navigation system. Several studies [31,32,33] state that the humeral component plays a key role in stability, deltoid efficiency and joint load. We believe that the planning of the humeral component can have a significant impact on achieving the right deltoid and conjoint tendon tension, with important implications for clinical outcomes.
To reduce this potential bias, we always performed an osteotomy at the level of the anatomical neck and used a size 0 insert in all implants.
A further limitation is certainly the small number of patients evaluated, although no studies involving larger populations are currently available in the literature.
Furthermore, the inability to control postoperative physiotherapy rehabilitation creates a significant variable, with its impact on the patient’s clinical course. In addition, when examining the time frame in which our study took place, it becomes apparent that a large proportion of the patients were operated during the Sars-Cov2 pandemic. This may have had an additional impact on the possibility of carrying out the rehabilitation course in the required manner and timeframe.
Conclusion
Navigation can be used as a reference to improve our knowledge of the impact that intraoperative parameters can have on the patient’s clinical outcome.
This technology could be a breakthrough to identify the range of lateralization of the glenoid implant, which is probably the most interesting data obtained from this study.
We observed that the patients with the best anteposition and abduction results had a lateralization range between 18 and 22 mm. When increasing the lateralization above 22 mm or reducing it below 18 mm, on the other hand, both the movements considered decreased in amplitude.
We therefore believe that further studies looking at larger samples of patients with long-term follow-up are needed to verify the true correlation between this range and the clinical outcomes achieved.
References
Sadoghi P, Vavken J, Leithner A, Vavken P (2015) Benefit of intraoperative navigation on glenoid component positioning during total shoulder arthroplasty. Arch Orthop Trauma Surg 135:41–47. https://doi.org/10.1007/s00402-014-2126-1
Boileau P (2016) Complications and revision of reverse total shoulder arthroplasty. Orthop Traumatol Surg Res 102:S33-43. https://doi.org/10.1016/j.otsr.2015.06.031
Cheung E, Willis M, Walker M, Clark R, Frankle MA (2011) Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg 19:439–449
Falaise V, Levigne C, Favard L, SOFEC (2011) Scapular notching in reverse shoulder arthroplasties: the influence of glenometaphyseal angle. Orthop Traumatol Surg Res OTSR 97:S131-137. https://doi.org/10.1016/j.otsr.2011.06.007
Frankle MA, Teramoto A, Luo Z-P, Levy JC, Pupello D (2009) Glenoid morphology in reverse shoulder arthroplasty: classification and surgical implications. J Shoulder Elbow Surg 18:874–885. https://doi.org/10.1016/j.jse.2009.02.013
Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA (2011) Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg 20:732–739. https://doi.org/10.1016/j.jse.2010.10.035
Lädermann A, Denard PJ, Boileau P, Farron A, Deransart P, Walch G (2018) What is the best glenoid configuration in onlay reverse shoulder arthroplasty? Int Orthop 42:1339–1346. https://doi.org/10.1007/s00264-018-3850-x
Lädermann A, Gueorguiev B, Charbonnier C, Stimec BV, Fasel JHD, Zderic I et al (2015) Scapular notching on kinematic simulated range of motion after reverse shoulder arthroplasty is not the result of impingement in adduction. Medicine (Baltimore) 94:e1615. https://doi.org/10.1097/MD.0000000000001615
Boileau P, Morin-Salvo N, Gauci M-O, Seeto BL, Chalmers PN, Holzer N et al (2017) Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management of glenoid bone loss and erosion. J Shoulder Elbow Surg 26:2133–2142. https://doi.org/10.1016/j.jse.2017.05.024
Boileau P, Moineau G, Roussanne Y, O’Shea K (2011) Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop 469:2558–2567. https://doi.org/10.1007/s11999-011-1775-4
Markes AR, Cheung E, Ma CB (2020) Failed reverse shoulder arthroplasty and recommendations for revision. Curr Rev Musculoskelet Med 13:1–10. https://doi.org/10.1007/s12178-020-09602-6
Goetti P, Denard PJ, Collin P, Ibrahim M, Mazzolari A, Lädermann A (2021) Biomechanics of anatomic and reverse shoulder arthroplasty. EFORT Open Rev 6:918–931. https://doi.org/10.1302/2058-5241.6.210014
Giorgini A, Tarallo L, Novi M, Porcellini G (2021) Computer-assisted surgery in reverse shoulder arthroplasty: early experience. Indian J Orthop 55:1003–1008. https://doi.org/10.1007/s43465-020-00344-8
Colasanti GB, Moreschini F, Cataldi C, Mondanelli N, Giannotti S (2020) GPS guided reverse shoulder arthroplasty. Acta Bio-Medica Atenei Parm 91:204–208. https://doi.org/10.23750/abm.v91i4-S.9377
Jahic D, Suero EM, Marjanovic B (2021) The use of computer navigation and patient specific instrumentation in shoulder arthroplasty: everyday practice, just for special cases or actually teaching a surgeon? Acta Inform Medica AIM J Soc Med Inform Bosnia Herzeg Cas Drustva Za Med Inform BiH 29:130–133. https://doi.org/10.5455/aim.2021.29.130-133
Burns DM, Frank T, Whyne CM, Henry PD (2019) Glenoid component positioning and guidance techniques in anatomic and reverse total shoulder arthroplasty: a systematic review and meta-analysis. Shoulder Elb 11:16–28. https://doi.org/10.1177/1758573218806252
Wang AW, Hayes A, Gibbons R, Mackie KE (2020) Computer navigation of the glenoid component in reverse total shoulder arthroplasty: a clinical trial to evaluate the learning curve. J Shoulder Elbow Surg 29:617–623. https://doi.org/10.1016/j.jse.2019.08.012
Erickson BJ, Chalmers PN, Denard P, Lederman E, Horneff G, Werner BC et al (2021) Does commercially available shoulder arthroplasty preoperative planning software agree with surgeon measurements of version, inclination, and subluxation? J Shoulder Elbow Surg 30:413–420. https://doi.org/10.1016/j.jse.2020.05.027
Werner BS, Hudek R, Burkhart KJ, Gohlke F (2017) The influence of three-dimensional planning on decision-making in total shoulder arthroplasty. J Shoulder Elbow Surg 26:1477–1483. https://doi.org/10.1016/j.jse.2017.01.006
Barrett I, Ramakrishnan A, Cheung E (2019) Safety and efficacy of intraoperative computer-navigated versus non-navigated shoulder arthroplasty at a tertiary referral. Orthop Clin North Am 50:95–101. https://doi.org/10.1016/j.ocl.2018.08.004
Gregory TM, Sankey A, Augereau B, Vandenbussche E, Amis A, Emery R et al (2013) Accuracy of glenoid component placement in total shoulder arthroplasty and its effect on clinical and radiological outcome in a retrospective, longitudinal, monocentric open study. PLoS ONE 8:e75791. https://doi.org/10.1371/journal.pone.0075791
Werner BS, Chaoui J, Walch G (2018) Glenosphere design affects range of movement and risk of friction-type scapular impingement in reverse shoulder arthroplasty. Bone Jt J 100-B:1182–1186. https://doi.org/10.1302/0301-620X.100B9.BJJ-2018-0264.R1
Kim S-J, Jang S-W, Jung K-H, Kim YS, Lee S-J, Yoo Y-S (2019) Analysis of impingement-free range of motion of the glenohumeral joint after reverse total shoulder arthroplasty using three different implant models. J Orthop Sci Off J Jpn Orthop Assoc 24:87–94. https://doi.org/10.1016/j.jos.2018.08.016
Keener JD, Patterson BM, Orvets N, Aleem AW, Chamberlain AM (2018) Optimizing reverse shoulder arthroplasty component position in the setting of advanced arthritis with posterior glenoid erosion: a computer-enhanced range of motion analysis. J Shoulder Elbow Surg 27:339–349. https://doi.org/10.1016/j.jse.2017.09.011
Berhouet J, Garaud P, Favard L (2014) Evaluation of the role of glenosphere design and humeral component retroversion in avoiding scapular notching during reverse shoulder arthroplasty. J Shoulder Elbow Surg 23:151–158. https://doi.org/10.1016/j.jse.2013.05.009
Rhee S-M, Lee JD, Park YB, Yoo JC, Oh JH (2019) Prognostic radiological factors affecting clinical outcomes of reverse shoulder arthroplasty in the Korean population. Clin Orthop Surg 11:112–119. https://doi.org/10.4055/cios.2019.11.1.112
Borbas P, Kriechling P, Fehler S, Bouaicha S, Wieser K (2021) The influence of Parkinson’s disease on outcome and complication rate of reverse total shoulder arthroplasty: a matched group analysis. Orthopedics 44:86–91. https://doi.org/10.3928/01477447-20210217-02
Jones RB, Greene AT, Polakovic SV, Hamilton MA, Mohajer NJ, Youderian AR et al (2020) Accuracy and precision of placement of the glenoid baseplate in reverse total shoulder arthroplasty using a novel computer assisted navigation system combined with preoperative planning: a controlled cadaveric study. Semin Arthroplasty JSES 30:73–82. https://doi.org/10.1053/j.sart.2020.05.004
Larose G, Greene AT, Jung A, Polakovic SV, Davis NZ, Zuckerman JD et al (2023) High intraoperative accuracy and low complication rate of computer-assisted navigation of the glenoid in total shoulder arthroplasty. J Shoulder Elbow Surg. https://doi.org/10.1016/j.jse.2022.12.021
Nguyen D, Ferreira LM, Brownhill JR, King GJW, Drosdowech DS, Faber KJ et al (2009) Improved accuracy of computer assisted glenoid implantation in total shoulder arthroplasty: an in-vitro randomized controlled trial. J Shoulder Elbow Surg 18:907–914. https://doi.org/10.1016/j.jse.2009.02.022
Lädermann A, Williams MD, Melis B, Hoffmeyer P, Walch G (2009) Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 18:588–595. https://doi.org/10.1016/j.jse.2009.03.012
Boileau P, Watkinson D, Hatzidakis AM, Hovorka I (2006) Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg 15:527–540. https://doi.org/10.1016/j.jse.2006.01.003
Ferrier A, Blasco L, Marcoin A, De Boissieu P, Siboni R, Nérot C et al (2018) Geometric modification of the humeral position after total reverse shoulder arthroplasty: what is the optimal lowering of the humerus? J Shoulder Elbow Surg 27:2207–2213. https://doi.org/10.1016/j.jse.2018.05.027
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The study was led according to the regulations established by the Clinical Research and Ethics Committee and to the Helsinki Declaration.
Ethical approval
No. AOU0013134/21.
Informed consent
Acquired for the study population.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tarallo, L., Giorgini, A., Micheloni, G. et al. Navigation in reverse shoulder arthroplasty: how the lateralization of glenosphere can affect the clinical outcome. Arch Orthop Trauma Surg 143, 5649–5656 (2023). https://doi.org/10.1007/s00402-023-04879-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-04879-x