Abstract
Introduction
Enhanced recovery after surgery (ERAS) leads to less morbidity, faster recovery, and, therefore, shorter hospital stays. The expected increment of primary total hip arthroplasty (THA) in the U.S. highlights the need for sufficient pain management. The favorable use of short-lasting spinal anesthesia enables early mobilization but may lead to increased opioid consumption the first 24 h (h) postoperatively.
Methods
In a retrospective study design, we compared conventional THA with postoperative immobilization for two days (non-ERAS) and enhanced recovery THA with early mobilization (ERAS group). Data assessment took place as part of the “Quality Improvement in Postoperative Pain Treatment project” (QUIPS). Initially, 2161 patients were enrolled, resulting in 630 after performing a matched pair analysis for sex, age, ASA score (American-Society-of-Anesthesiology) and preoperative pain score. Patient-reported pain scores, objectified by a numerical rating scale (NRS), opioid consumption and side effects were evaluated 24 h postoperatively.
Results
The ERAS group revealed higher activity-related pain (p = 0.002), accompanied by significantly higher opioid consumption (p < 0.001). Maximum and minimum pain as well as side effects did not show significant differences (p > 0.05).
Conclusion
This study is the first to analyze pain scores, opioid consumption, and side effects in a matched pair analyses at this early stage and supports the implementation of an ERAS concept for THA. Taking into consideration the early postoperative mobilization, we were not able to detect a difference regarding postoperative pain. Although opioid consumption appeared to be higher in ERAS group, occurrence of side effects ranged among comparable percentages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leading to less morbidity, faster recovery and, therefore, shorter hospital stays, enhanced recovery after surgery programs (ERAS) experience growing acceptance and worldwide adoption [22, 28, 35]. Being initially established for colorectal operations, ERAS gained more and more importance in orthopedic surgery [21, 23]. Big orthopedic operations such as primary total hip arthroplasty (THA) are accompanied by a resulting pathophysiologic catabolism and, therefore, long recovery. ERAS programs effectively reduce catabolism with reduced loss of muscular strength as well as less thromboembolic and gastrointestinal adverse reactions [3, 23, 24].
Due to demographic change, current predictions expect an incremental rise of primary THA in the U.S. by 284% in 2040, reaching a preliminary peak of 1.429.000 procedures annually [34]. Although primary THA was named the most successful operation of the century, about 10% of patients report postoperative dissatisfaction [16, 18, 26]. The main reason for postoperative dissatisfaction lies in chronic pain, followed by limitation of function [16]. Therefore, targeted multimodal pain management is of fundamental importance to inhibit the development of chronic pain syndromes [7, 14]. Spinal anesthesia as well as the combination of spinal and general anesthesia showed lower postoperative pain scores compared to general anesthesia [15]. Different studies report the advantages of ERAS concepts in total joint arthroplasty [11, 13, 22, 25]. A recent study compared the postoperative functional outcome and quality of life of patients receiving primary THA with an ERAS concept and such receiving conventional THA. Patients in the ERAS group reported superior functional results in the WOMAC score 1 year postoperatively [27]. Nevertheless, literature review reveals inconsistent data availability concerning early postoperative pain [17, 19, 39]. While many studies aim on reduction of the length of hospital stay as the main indicator for success, climaxing in same day discharge, the early pain, and complications 24 h postoperatively can mostly not be evaluated due to early discharge [19, 39].
The beneficial use of short-lasting spinal anesthesia in ERAS concepts enables early mobilization [22]. Due to its short-lasting effect with efficacy for only up to 2–4 h, one might expect a higher oral opioid consumption in the first 24 h postoperatively which may result in increased rates of side effects. Even though some studies report lesser opioid consumption in fast-track surgery, no study compared opioid consumption and occurrence of side effects in a matched pair analyses at that early stage [31].
Aim of the study
In a matched pair analysis of 630 patients, comparing conventional with enhanced recovery THA with early mobilization, we aimed to evaluate patient-reported pain, opioid consumption, and side effects 24 h postoperatively.
Methods
Data assessment took place between 2009 and 2021. In a retrospective study design, in total, 2161 patients receiving primary THA at our university hospital were included. Criteria for inclusion were cementless primary THA and fully orientated patients, older than 18 years. Patients with BMI > 40 kg/m2, immobilization in a wheelchair or need for a wheeled walker were excluded. Furthermore, refusal to participate, disorientation, sedation, visitors during data assessment or cognitive dysfunction represented criteria for exclusion. An independent, special trained pain nurse interviewed the patients 24 h postoperatively. A validated 16-item questionnaire was used, asking for minimal and maximum pain since surgery on a numerical rating scale (NRS, 0 = no pain, 10 = worst imaginable pain) to document postoperative pain management and occurrence of complications. Satisfaction with pain management and participation in pain management was objectified by an inversed NRS (0 = completely dissatisfied, 10 = perfectly satisfied). The file reporting demographic data as well as the patients’ questionnaire is provided under the following URL: https://www.quips-projekt.de/services/dateien and attached to the document.
Patients in both groups received primary THA via a modified Watson–Jones approach without transection of muscular tissue [6]. Patients were placed in lateral position and an anterolateral mini-incision was performed. Using the intermuscular plane between tensor fascia lata and gluteus medius, the integrity of the muscles is preserved while the intactness of the posterior capsule prevents posterior dislocation [6]. Postoperative pain management was identical in both groups based upon WHO three-step analgesic ladder [1, 8]. Consisting of three steps which range from mild to severe pain, it is well established and widely used. First step describes mild pain and is treated by nonsteroidal anti-inflammatory drugs (NSAIDs) without adjuvants. In step two, additional weak opioids are used, while severe pain, representing step three, is treated by additional potent opioids [1, 8].
The established ERAS concept for primary THA consisted of local infiltration analgesia, special anesthesia, and a targeted postoperative treatment protocol: Patients received education before the operation in terms of pain management and gait training by physiotherapists. They were trained to walk with crutches in advance and educated what the limitations in mobilization they have to expect directly after the operation. One hour preoperatively, participants were administered a non-steroid-anti-inflammatory-drug (etoricoxib 90 mg). Every patient received a short-lasting spinal anesthesia (4 ml prilocaine 1%, hyperbaric and 10 µg sufentanil) combined with the intravenous application of dexamethasone (8 mg). In addition, patients received tranexamic acid intravenously (1 g) as well as topically (2 g). Intraoperatively local infiltration analgesia (ropivacaine 200 mg, adrenaline 0.5 mg) was administered periarticular, femoral, acetabular, and subcutaneously. No drains were placed in the operating field. Patients were immediately allowed full weight-bearing and first mobilization was carried out 2–3 h postoperatively at an Intermediate Care Unit (IMC). Every patient received intensive physiotherapy by a special trained physiotherapist for half an hour two times a day. Patients were encouraged to use a special developed exercise course with the aim to improve range of motion and balance.
In contrast to the ERAS concept, patients receiving conventional primary THA (non-ERAS) received neither gait training, nor analgesic medication directly before the operation. A long-lasting spinal anesthesia was performed (4 ml bupivacaine, 0.5%). Intraoperatively, neither local infiltration analgesia, nor tranexamic acid or dexamethasone was administered. Wound drains were consequently applied. At the earliest, patients were mobilized for the first time the day after the operation. Postoperative partial weight-bearing was allowed. Physiotherapy took place once a day.
Data assessment
Data acquisition was performed as part of the Quality Improvement in Postoperative Pain Treatment project (QUIPS), a nationwide German benchmarking initiative for postoperative pain [30]. Including data sets from over 600.000 patients and over 200 participating hospitals, it demonstrates the largest database for acute postoperative pain worldwide. The project is supported by the German Society of Anesthesiologist and the German Society of Surgeons [29]. All data were anonymized.
The present study was conducted in agreement with the ethical standards of the Declaration of Helsinki (1975). A member of the study crew informed every patient orally as well as in written form about the study. Every participant signed the informed consent before enrollment. Participation was voluntary with possible withdrawal at any time. The ethics committee as well as the data security board of the Jena University Hospital (Jena, Germany) approved the data acquisition for the QUIPS project. The study is registered in the DRKS with number DRKS00006153 (WHO register).
Statistical analysis
To ensure groups of identical size and cofounders, we performed a 1:1 matched pair analysis, based upon gender, age, ASA, and chronic NRS preoperatively [27]. If there was more than one possible matching partner, the matching patient was chosen randomly. Metric variables are noted as median ± interquartile range (IQR). Categorical variables are noted in relative frequencies. Shapiro–Wilk Normality Test was used to test for normal distribution. Data were not normally distributed. To test for statistical significance, we used Chi-square test and nonparametric Mann–Whitney U. Statistical significance was considered at p < 0.05. Statistically significant data are noted in italics. Statistical analysis was performed with SPSS (IBM SPSS Statistics 28, International Business Machines Corporation (IBM), Armonk, New York, U.S.).
Results
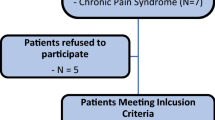
Between 11/2009 and 11/2021, 1843 patients received conventional primary THA, while 318 patients received THA featuring the described enhanced recovery concept. After performing a 1:1 matched pair analysis for gender, age, ASA, and NRS preoperatively, each group consisted of 315 patients. The flowchart is illustrated in Fig. 1.
As result of the matched pair analysis, demographic data of gender, age, ASA, and preoperative pain are almost identical. The duration of surgery appeared to be significantly shorter in the ERAS group than in conventional THA (p = 0.002, Table 1). Occurrence of chronic pain preoperatively did not reveal significant differences. Significantly more patients in Non-ERAS group had chronic pain not only in the operated region (p = 0.032). Demographic data are noted in Table 1.
Pain development and functional outcome
Evaluation of minimum and maximum pain as well as satisfaction and patients’ participation in pain management did not show significant differences between the two concepts 24 h postoperatively (p > 0.05, Table 2). The ERAS group revealed significantly higher pain scores in activity-related pain (p = 0.002, Table 2). Figure 2 shows the boxplots of the two groups for activity-related pain.
The functional outcome revealed identical results and the ERAS group showed a significantly higher percentage of pain affected ability to move (p = 0.019). The other functional outcome parameters ranged among comparable values in both groups (p > 0.05, Table 3).
Oral opioid consumption and side effects
Patients in the ERAS group revealed significantly higher demand for oral opioids at the IMC unit as well as at ward than the conventional THA group (both p < 0.001). The data are noted in Table 4. Figure 3 illustrates the comparison between both groups in regards to the relative frequency of opioid consumption.
The evaluation of the occurrence of side effects did not show significant differences between both groups (p > 0.05, Table 5).
Discussion
This study is the first to analyze pain scores, opioid consumption, and side effects after primary THA at this early stage, comparing conventional THA and an ERAS concept. Although opioid consumption and pain scores were significantly higher within 24 h after surgery, it supports the implementation of an ERAS concept as the advantages like less morbidity and faster recovery predominate.
The comparison between conventional THA and an ERAS concept with early mobilization revealed significantly higher activity-related pain as well as higher opioid consumption in the ERAS group the first 24 h postoperatively. It must be taken into consideration that patients receiving conventional THA were first mobilized more than 24 h after surgery, accordingly after the data assessment. Hence, patients in the conventional THA group did not experience early mobilization and, therefore, did not undergo activity-related pain the first 24 h. In contrast, patients in the ERAS group were exposed to early mobilization as early as 2 h after surgery at an IMC unit. The pain scores after 24 h postoperatively were not imposed; therefore, it is not possible to make a final statement on further opioid usage. Nevertheless, the opioid usage on ward was significantly less than on IMC.
In addition, although the ERAS group reported significantly more activity-related pain, the difference is not that big and might be from minor clinical importance (NRS activity related: ERAS 3 ± 2 vs. non-ERAS 2 ± 2). Farrar et al. defined a change of at least 2 points on an NRS as clinically relevant [12]. Therefore, a statistically significant difference of one point on an NRS might be of minor clinical relevance for the patients. This assumption is indirectly supported by absence of a significant difference in maximum and minimum pain between the two groups (both p > 0.05). In summary, taking into consideration the early postoperative mobilization, we were not able to detect a difference regarding postoperative pain. Even though there is statistically difference it might not be clinically different.
Compared to conventional THA, opioid consumption in ERAS group at IMC unit almost doubled. At ward, opioid demand still appeared to be higher than in the non-ERAS group, though less distinctive. One explanation might be the long-lasting effect of opioid spinal anesthesia in conventional THA, in contrast to the short-lasting one in the ERAS group. While the difference is more pronounced at IMC unit, the effect of anesthesia is fading, and it already harmonized more at ward. As described above, the additional stress and pain through early mobilization in the ERAS group have to be taken into consideration and might represent the true cause for the measured difference.
Another reason for the high opioid consumption within the first 24 h postoperatively may be the occurrence of rebound pain after regional anesthesia. The phenomenon of rebound pain as a side effect of regional anesthesia was first reported in 2005; nevertheless, its causes are still mainly unclear [37]. It is described as a disproportional excessive pain as the effect of local anesthesia fades away after primary good perioperative pain compensation [36, 37]. It is discussed controversially in literature with prevalence of severe rebound pain in up to 40% of patients [10, 38]. Despite its high occurrence, studies report high rates of patient satisfaction [4]. In an analysis of possible prevention strategies, Dawson et al. supported a multimodal therapy concept with wider use of opioids [10].
Some studies report a significantly higher occurrence of dizziness, nausea, and vomiting in patients receiving fast-track surgery [2, 20]. In contrast, the evaluation of postoperative side effects did not show significant differences between both groups. Even though opioid demand appeared to be significantly higher in the ERAS group, possible side effects such as nausea, vomiting, and tiredness showed comparable values.
The duration of surgery was significantly shorter in the ERAS group (p = 0.002). Extended surgery time demonstrates a major risk factor for infections and postoperative complications like delirium [32, 33]. Moreover, operation time is expensive, representing an economical factor to reduce hospital costs [9]. Nevertheless, reduction of surgery time should not be seen as the primary goal, according to the fast-track protocol principle “first better—then faster” [40, 41]. Nevertheless, we were able to disprove the fear of an extended surgery time through additional interventions such as generous local infiltration anesthesia in an ERAS concept. This study did not focus on shortening the hospital stay or on evaluating the readmission rate, as it has been already described, and proved in several publications [5, 22, 25].
The present study features some limitations. First, the study followed a retrospective design. For better comparison we performed a matched pair analysis for sex, age, ASA, and preoperative pain scores to reduce covariate bias. Second, data acquisition only took place at one instance without further assessments and the evaluation of progress is thus missing with regards to the QUIPS-projects study design. Third, there may be a potential selection bias through the novel establishment of the ERAS concept at our university hospital.
Conclusion
Although the biggest differences in pain and opioid consumption are expected the first 24 h after the operation, the present study is the first to analyze this in depth in a matched pair analysis. Taking into consideration the early mobilization, we did not detect a difference in postoperative pain comparing conventional THA and THA with an ERAS concept. Although opioid consumption appeared to be significantly higher in the ERAS group, the occurrence of side effects ranged among comparable percentages. In addition, the operation time in the ERAS group was significantly shorter. In summary, this study supports the implementation of an ERAS concept for primary THA.
Data availability
On request, data are available at the authors’ institution.
References
Anekar AA, Cascella M (2022) WHO analgesic ladder. In: StatPearls. Publishing LLC, Treasure Island
Antrobus JD, Bryson GL (2011) Enhanced recovery for arthroplasty: good for the patient or good for the hospital? Can J Anaesth 58(10):891–896. https://doi.org/10.1007/s12630-011-9564-9
Bandholm T, Kehlet H (2012) Physiotherapy exercise after fast-track total hip and knee arthroplasty: time for reconsideration? Arch Phys Med Rehabil 93(7):1292–1294. https://doi.org/10.1016/j.apmr.2012.02.014
Barry GS, Bailey JG, Sardinha J, Brousseau P, Uppal V (2021) Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br J Anaesth 126(4):862–871. https://doi.org/10.1016/j.bja.2020.10.035
Berg U, BuLow E, Sundberg M, Rolfson O (2018) No increase in readmissions or adverse events after implementation of fast-track program in total hip and knee replacement at 8 Swedish hospitals: an observational before-and-after study of 14,148 total joint replacements 2011–2015. Acta Orthop 89(5):522–527. https://doi.org/10.1080/17453674.2018.1492507
Bertin KC, Röttinger H (2004) Anterolateral mini-incision hip replacement surgery: a modified Watson-Jones approach. Clin Orthop Relat Res 429:248–255
Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P (2012) What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2(1):e000435. https://doi.org/10.1136/bmjopen-2011-000435
Bucher M (2017) WHO-Stufenschema. In: Pschyrembel Online. Walter de Gruyter GmbH, Berlin. https://www.pschyrembel.de/WHO-Stufenschema/K0PQ7/doc/. Accessed 24 Mar 2022
Childers CP, Maggard-Gibbons M (2018) Understanding costs of care in the operating room. JAMA Surg 153(4):e176233. https://doi.org/10.1001/jamasurg.2017.6233
Dawson S, Loewenstein SN (2021) Severe rebound pain after peripheral nerve block for ambulatory extremity surgery is an underappreciated problem. Comment on Br J Anaesth 2021;126:862–71. Br J Anaesth 126(6):e204–e205. https://doi.org/10.1016/j.bja.2021.02.017
den Hartog YM, Mathijssen NM, Vehmeijer SB (2013) Reduced length of hospital stay after the introduction of a rapid recovery protocol for primary THA procedures. Acta Orthop 84(5):444–447. https://doi.org/10.3109/17453674.2013.838657
Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole MR (2001) Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 94(2):149–158. https://doi.org/10.1016/S0304-3959(01)00349-9
Frassanito L, Vergari A, Nestorini R et al (2020) Enhanced recovery after surgery (ERAS) in hip and knee replacement surgery: description of a multidisciplinary program to improve management of the patients undergoing major orthopedic surgery. Musculoskelet Surg 104(1):87–92. https://doi.org/10.1007/s12306-019-00603-4
Glare P, Aubrey KR, Myles PS (2019) Transition from acute to chronic pain after surgery. Lancet 393(10180):1537–1546. https://doi.org/10.1016/S0140-6736(19)30352-6
Greimel F, Maderbacher G, Zeman F, Grifka J, Meissner W, Benditz A (2017) No clinical difference comparing general, regional, and combination anesthesia in hip arthroplasty: a multicenter cohort-study regarding perioperative pain management and patient satisfaction. J Arthroplasty 32(11):3429–3433. https://doi.org/10.1016/j.arth.2017.05.038
Halawi MJ, Jongbloed W, Baron S, Savoy L, Williams VJ, Cote MP (2019) Patient dissatisfaction after primary total joint arthroplasty: the patient perspective. J Arthroplasty 34(6):1093–1096. https://doi.org/10.1016/j.arth.2019.01.075
Hardy A, Courgeon M, Pellei K, Desmeules F, Loubert C, Vendittoli PA (2022) Improved clinical outcomes of outpatient enhanced recovery hip and knee replacements in comparison to standard inpatient procedures: a study of patients who experienced both. Orthop Traumatol Surg Res. https://doi.org/10.1016/j.otsr.2022.103236
Heath EL, Ackerman IN, Cashman K, Lorimer M, Graves SE, Harris IA (2021) Patient-reported outcomes after hip and knee arthroplasty : results from a large national registry. Bone Jt Open 2(6):422-432. https://doi.org/10.1302/2633-1462.26.Bjo-2021-0053.R1
Hoffmann JD, Kusnezov NA, Dunn JC, Zarkadis NJ, Goodman GP, Berger RA (2018) The shift to same-day outpatient joint arthroplasty: a systematic review. J Arthroplasty 33(4):1265–1274. https://doi.org/10.1016/j.arth.2017.11.027
Husted H, Lunn TH, Troelsen A, Gaarn-Larsen L, Kristensen BB, Kehlet H (2011) Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop 82(6):679–684. https://doi.org/10.3109/17453674.2011.636682
Kehlet H (2008) Fast-track colorectal surgery. Lancet 371(9615):791–793. https://doi.org/10.1016/S0140-6736(08)60357-8
Kehlet H (2013) Fast-track hip and knee arthroplasty. Lancet 381(9878):1600–1602. https://doi.org/10.1016/S0140-6736(13)61003-X
Kehlet H, Dahl JB (2003) Anaesthesia, surgery, and challenges in postoperative recovery. Lancet 362(9399):1921–1928. https://doi.org/10.1016/S0140-6736(03)14966-5
Kehlet H, Wilmore DW (2008) Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 248(2):189–198. https://doi.org/10.1097/SLA.0b013e31817f2c1a
Khan SK, Malviya A, Muller SD et al (2014) Reduced short-term complications and mortality following enhanced recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop 85(1):26–31. https://doi.org/10.3109/17453674.2013.874925
Learmonth ID, Young C, Rorabeck C (2007) The operation of the century: total hip replacement. Lancet 370(9597):1508–1519. https://doi.org/10.1016/s0140-6736(07)60457-7
Leiss F, Schindler M, Gotz JS et al (2021) Superior functional outcome and comparable health-related quality of life after enhanced recovery vs. conventional THA: a retrospective matched pair analysis. J Clin Med 10(14):3096. https://doi.org/10.3390/jcm10143096
Malviya A, Martin K, Harper I et al (2011) Enhanced recovery program for hip and knee replacement reduces death rate. Acta Orthop 82(5):577–581. https://doi.org/10.3109/17453674.2011.618911
Meissner W, Mescha S, Rothaug J et al (2008) Quality improvement in postoperative pain management: results from the QUIPS project. Dtsch Arztebl Int 105(50):865–870. https://doi.org/10.3238/arztebl.2008.0865
Meissner W, Ullrich K, Zwacka S (2006) Benchmarking as a tool of continuous quality improvement in postoperative pain management. Eur J Anaesthesiol 23(2):142–148. https://doi.org/10.1017/s026502150500205x
Noth U, Geiser T, Kranich T et al (2019) Fast track strategies in hip arthroplasty. Orthopade 48(4):330–336. https://doi.org/10.1007/s00132-019-03697-7
Orland MD, Lee RY, Naami EE, Patetta MJ, Hussain AK, Gonzalez MH (2020) Surgical duration implicated in major postoperative complications in total hip and total knee arthroplasty: a retrospective cohort study. J Am Acad Orthop Surg Glob Res Rev 4(11):e20.00043. https://doi.org/10.5435/JAAOSGlobal-D-20-00043
Ravi B, Pincus D, Choi S, Jenkinson R, Wasserstein DN, Redelmeier DA (2019) Association of duration of surgery with postoperative delirium among patients receiving hip fracture repair. JAMA Netw Open 2(2):e190111. https://doi.org/10.1001/jamanetworkopen.2019.0111
Singh JA, Yu S, Chen L, Cleveland JD (2019) Rates of total joint replacement in the United States: future projections to 2020–2040 using the national inpatient sample. J Rheumatol 46(9):1134–1140. https://doi.org/10.3899/jrheum.170990
Stambough JB, Nunley RM, Curry MC, Steger-May K, Clohisy JC (2015) Rapid recovery protocols for primary total hip arthroplasty can safely reduce length of stay without increasing readmissions. J Arthroplasty 30(4):521–526. https://doi.org/10.1016/j.arth.2015.01.023
Stone A, Lirk P, Vlassakov K (2022) Rebound pain after peripheral nerve blockade-bad timing or rude awakening? Anesthesiol Clin 40(3):445–454. https://doi.org/10.1016/j.anclin.2022.03.002
Streb T, Schneider A, Wiesmann T et al (2022) „Rebound pain“ – von der Definition bis zur Therapie. Anaesthesiologie 71(8):638–645. https://doi.org/10.1007/s00101-022-01120-z
Sunderland S, Yarnold CH, Head SJ et al (2016) Regional versus general anesthesia and the incidence of unplanned health care resource utilization for postoperative pain after wrist fracture surgery: results from a retrospective quality improvement project. Reg Anesth Pain Med 41(1):22–27. https://doi.org/10.1097/aap.0000000000000325
Vendittoli P-A, Pellei K, Desmeules F et al (2019) Enhanced recovery short-stay hip and knee joint replacement program improves patients outcomes while reducing hospital costs. Orthop Traumatol Surg Res 105(7):1237–1243. https://doi.org/10.1016/j.otsr.2019.08.013
Wainwright TW, Gill M, McDonald DA et al (2020) Consensus statement for perioperative care in total hip replacement and total knee replacement surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. Acta Orthop 91(1):3–19. https://doi.org/10.1080/17453674.2019.1683790
Wainwright TW, Kehlet H (2019) Fast-track hip and knee arthroplasty—have we reached the goal? Acta Orthop 90(1):3–5. https://doi.org/10.1080/17453674.2018.1550708
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the interpretation of data and were involved in drafting the manuscript. JR, MS, FL, FG, AB, JG: made substantial contributions to the conception and design of the study. JR, FG, AB: participated in the acquisition of data, analysis, and statistics. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Institutional review board
The ethics committee as well as the data security board of Jena University Hospital (Jena, Germany) approved the project. Study registration in the DRKS (DRKS00006153, WHO register).
Informed consent
The study was carried out in accordance with the ethical standards of the Declaration of Helsinki of 1975. The study crew informed patients in written form as well as orally. Written informed consent was obtained from all participants before enrollment. Participation was voluntary with possible withdrawal at any time.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reinhard, J., Schindler, M., Leiss, F. et al. No clinically significant difference in postoperative pain and side effects comparing conventional and enhanced recovery total hip arthroplasty with early mobilization. Arch Orthop Trauma Surg 143, 6069–6076 (2023). https://doi.org/10.1007/s00402-023-04858-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-04858-2