Abstract
Introduction
Augmented anterior cruciate ligament reconstruction (ACLR) techniques have been proposed to reduce the high reported re-injury rates and low rates of return to sport (RTS). This study reports clinical outcomes, RTS and re-injury rates in patients undergoing ACLR using autologous hamstrings augmented with suture tape.
Materials and methods
A total of 53 patients were prospectively recruited, undergoing ACLR using hamstrings with suture tape augmentation, combined with a structured rehabilitation programme. Outcomes were collected to 24 months, including patient-reported outcome measures (PROMs), KT-1000 measurements, peak isokinetic knee strength and a four hop test battery. Limb Symmetry Indices (LSIs) were calculated for performance measures, whilst RTS rates, re-tears and re-operations were presented.
Results
There were no significant side-to-side differences in anterior tibial translation between the operated and non-operated knees at 6 months (p = 0.433), with no increase (p = 0.841) in side-to-side anterior tibial translation from 6 to 24 months. At 24 months, 98.0% of patients demonstrated normal (< 3 mm) or near normal (3–5 mm) side-to-side differences. LSIs for peak knee extensor torque (p < 0.0001) and the single (p = 0.001), triple (p = 0.001) and triple crossover (p < 0.0001) hop tests for distance significantly improved. All PROMs significantly improved (p < 0.0001), with 70.2% and 85.7% of patients actively participating in pivoting sports at 12 and 24 months, respectively. Three patients underwent secondary procedures for meniscal symptoms. One patient suffered an ACL re-tear (17 months), with no further ipsilateral or contralateral injuries.
Conclusion
ACLR with suture tape augmentation demonstrated no evidence of excessive anterior tibial translation, high-scoring PROMs, sound performance scores, a high rate of RTS and low re-injury rate.
Similar content being viewed by others
Introduction
Anterior cruciate ligament reconstruction (ACLR) is common [1] and, whilst a primary post-operative goal for many patients is a return to sport (RTS), it has been reported that across all patients, only 65% of patients return to their pre-injury level of sport [2]. Furthermore, an overall secondary re-injury rate of 7% has been reported, along with an 8% incidence of contralateral ACL tear, with a combined (ipsilateral and contralateral) ACL injury rate of 23% specifically in patients < 25 years of age who do RTS [3]. The reasons for re-injury are multifactorial [4], though a recent systematic review reported no significant differences in graft failure rates across varied graft types (quadriceps, hamstring and patellar tendon autografts, or allografts) [5]. In addition to ensuring that strength and functional performance is best restored given their link with re-injury risk [6, 7], surgical reconstruction techniques involving autograft (or allograft) augmentation have been proposed [8,9,10,11,12,13] in an attempt to improve outcomes and reduce re-injury rates. ACLR augmentation may permit early ACL reinforcement and graft stability prior to graft incorporation, also expediting post-operative recovery and accelerating rehabilitation [9, 14].
A range of augmented procedures and devices have been reported [15]. Encouraging clinical and RTS outcomes have been more recently reported when using a LARS ligament (LARS, Ligament Augmentation Reconstruction System, Corin Pty. Ltd.) to augment a hamstrings autograft [13, 16, 17], with patient outcomes of those undergoing augmented ACLR better than those undergoing non-augmented ACLR [16]. However, earlier use of synthetic augmentation, including LARS, appeared to present with excessive synovitis and in higher ACL graft failure rates [18,19,20,21,22,23,24,25]. A more recently employed device to augment an ACLR is FiberTape® (Arthrex, Naples, Florida, USA) [8, 12, 14, 26], with a retrospective comparison of outcomes in patients undergoing ACLR with and without suture augmentation with FiberTape® demonstrating improved outcomes with augmentation [14]. However, studies using FiberTape® augmentation are limited and a greater number of published papers exist related to the use of FiberTape® reinforcement in the context of ACL repair [27,28,29], rather than reconstruction, although even then many of these are technical notes and not studies reporting patient outcomes.
This study presents the clinical outcomes of a prospective patient cohort undergoing ACLR employing autologous hamstrings augmented with suture tape, combined with a progressive, structured rehabilitation programme. With the aforementioned reported re-injury and RTS rates in mind, it was hypothesized that: (1) no significant post-operative differences in anterior tibial translation would exist between the operated and non-operated limbs, (2) a low re-injury rate (< 5%) would be observed over the 24-month period, (3) a high RTS rate (> 70%) would be observed at 12 and 24 months and (4) a significant improvement in patient-reported outcome measures (PROMs) and objective outcomes would be observed following surgery.
Material and methods
Patients
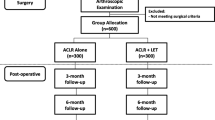
Between March 2018 and November 2019, 57 patients scheduled for ACLR employing a hamstrings autograft and augmented with a suture tape were referred by a single surgeon in a private orthopaedic clinic for study discussion, recruitment and subsequent pre-operative review, of which 53 patients elected to participate (Fig. 1, Level IV prospective case series). Patients were candidates for surgery based on history, current symptoms and orthopaedic clinical examination, whilst magnetic resonance imaging (MRI) confirmed the ACL rupture in all patients. Patients were invited to participate in the study if they were deemed candidates for surgery, were 16–50 years of age (and skeletally mature) and required an isolated primary ACLR, with or without concomitant meniscal surgery. Whilst not encountered, patients were excluded from study participation if they presented with a body mass index (BMI) ≥ 40 or were unwilling or unable to participate in the post-operative rehabilitation protocol (outlined below). Ethics approval was provided by the relevant Human Research Ethics Committee (HREC) and the written consent of all participants was obtained prior to review.
The surgical technique
All surgeries were performed by the senior author. Examination under anaesthesia was performed prior to tourniquet application to assess laxity of the injured ACL knee in comparison to the contralateral knee and clinically confirm a rupture of the ACL. Knee arthroscopy was subsequently performed to confirm the clinical diagnosis and further evaluate concomitant and/or chondral damage, which was addressed initially if required. Unstable ACL remnant tissue was then removed.
The ACL tunnels were routinely dictated by the anatomical positions of the existing ACL remnants. The tibial footprint of the ACL was initially identified, and all unstable remnant was removed. The tibial jig was placed centrally in the tibial footprint, and the tibial tunnel was prepared within the centre of the tibial ACL remnant (Fig. 2). Femoral tunnel preparation was performed in a similar way. The femoral anteromedial bundle soft tissue footprint was identified and an awl mark was created. A secondary check was via confirming a prepared tunnel position 2-4 mm off the posterior notch wall, generally in the 2.00 o’clock (left knee) or 10.00 o’clock (right knee) position (Fig. 3), with femoral tunnels drilled in maximal knee flexion. The ACL tibial remnant was cleared from the tibia to allow unobstructed passage of the graft within the knee.
Semitendinosus and gracilis tendons were harvested from the ipsilateral knee through a 2–3 cm transverse incision approximately 1 cm above the pes anserinus, and prepared as doubled grafts. The combined diameter was measured to establish bone tunnel size reaming, with a minimum graft diameter of 8 mm confirmed for all cases. The harvested hamstring grafts were then passed through the ACL TightRope RT (Arthrex, Naples, Florida, USA) implant loop of the suspensory button creating a 4-strand hamstring graft. A FiberTape® (Arthrex, Naples, Florida, USA) was then attached by a half hitch to the femoral button to act as a ‘seat belt’ augmentation of the graft construct, creating a two-strand internal brace that was essentially placed alongside the autograft (Fig. 4).
The graft was passaged after placing a suture via a shuttle technique from the tibia through to the button tunnel on the femur. The graft was seated with maximal manual tension whilst cycling the knee ten times. The tibial fixation was performed with a peek interference screw (Arthrex, Naples, Florida, USA), 1 mm larger than the tunnel and positioned in full knee extension. The two internal brace strands were fixed in an accessory position with a knotless anchor 1 cm distal to the tibial tunnel. The knee was place in full extension and the tight rope femoral suture was toggled to optimize maximum graft tension. The final graft construct is shown in Fig. 5.
Rehabilitation
A standardized rehabilitation programme was implemented for all patients, aiming for a supervised therapist session every 2 weeks (starting from 2 weeks post-surgery) for the first 5–6 months (12 supervised sessions in total), with ongoing periodic review beyond 6 months post-surgery as required. These sessions were supplemented with an independent home and/or gym-based programme, aiming for 2–3 sessions in total per week. Whilst the home/gym-based programme was not closely monitored, 88.7% (47 of 53) of patients attended ≥ 75% of the designated supervised sessions, with the remaining 11.3% (6 of 53) of patients attending 58–67% of the designated sessions. This was generally due to geographical location and/or COVID-19 restrictions, and these patients were more closely monitored from afar as needed. All supervised rehabilitation was undertaken in a single, private out-patient therapy clinic. Table 1 provides an overview of the programme implemented. In brief, early post-operative management included weight bearing as tolerated, early circulatory (such as foot/ankle pumps) and knee range of motion (ROM) exercises, followed by a progressive programme aiming to restore strength and load capacity, with progression towards running and activities that better prepared the patient for an eventual RTS.
Whilst late-stage progression through sport-specific training-based activities was also dependent on the patient’s specific sport, these aspects were not documented as part of the current patient cohort and patients transitioned through these components of training at their own discretion in collaboration with their sporting team. Whilst RTS was not advised until ≥ 9 months post-surgery and patients were counselled on specific objective criteria that should be attained before returning to sports activities (such as the restoration active knee extension ROM and flexion ROM LSI ≥ 90%, ≥ 90% LSI in hop tests and peak isokinetic knee extensor and flexor strength), this was not enforced and still largely at the final discretion of the patient.
Patient assessment
First, all patients underwent a formal knee laxity exam performed in the clinic by the senior author (PA) at 4 months post-surgery, specifically to assess rotatory laxity grading via pivot shift evaluation. Anterior tibial translation (mm) was measured on both knees during a maximal manual test (MMT) using the KT-1000 knee arthrometer (MEDmetric Corp., San Diego, CA, USA) at 6, 9, 12 and 24 months post-surgery. Active knee flexion and extension range of motion (ROM, degrees) using a hand-held long-arm goniometer was assessed on the operated limb at 6 weeks, as well as 4, 6, 9, 12 and 24 months post-surgery. Patients underwent a 4-hop battery and assessment of peak isokinetic knee extensor and flexor strength (Nm) at 6, 9, 12 and 24 months. The 4-hop battery included the single hop for distance (SHD, m), the 6 m timed hop (6MTH, s), the triple hop for distance (THD, m) and the triple crossover hop for distance (TCHD, m) [30]. Peak isokinetic knee extensor and flexor strength was measured at 90°/s, using an isokinetic dynamometer (Isosport International, Gepps Cross, South Australia). These reviews and all nominated assessments (apart from the laxity exam undertaken by the senior author at 4 months) were undertaken by a qualified therapist, with 20 years of experience undertaking all of the aforementioned assessments.
Several patient-reported outcome measures (PROMs) were undertaken pre-surgery and at various post-operative time-points. These included the International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form [31], the Knee Outcome Survey (KOS) Activities of Daily Living Scale [32], the Cincinnati Knee Rating System (CKRS) [33], the Lysholm Knee Score (LKS) [34], the Tegner Activity Scale (TAS) [35], the Anterior Cruciate Ligament Return to Sport after Injury (ACL-RSI) [36] and the Noyes Sports Activity Rating Scale (NSARS) [37]. A satisfaction score was employed at 24 months post-surgery, evaluating patient satisfaction with the surgery overall, as well as with the surgery to relieve pain, improve the ability to perform normal daily and work activities, improve the ability to return to recreational activities (including walking, swimming, cycling, golf, dancing), and improve the ability to participate in sport (including sports such as tennis, netball, soccer and football). A Likert Response Scale was employed with descriptors Very Satisfied, Somewhat Satisfied, Somewhat Dissatisfied and Very Dissatisfied.
Data and statistical analysis
For this prospective study, a priori sample size power calculation was determined based on the recommendations of Cohen [38] and employing data previously collected and published in patients undergoing ACLR with a hamstrings autograft, augmented with LARS [13]. Therefore, in using this existing data and for an anticipated moderate effect size (d = 0.67) in the primary outcome (anterior tibial translation as evaluated via side-to-side difference in anterior tibial translation in mm for the KT-1000 at 6 months post-surgery), assuming an SD of 3 mm and at alpha level of 0.05 and a power of 0.9, the sample size was estimated at 49 patients to demonstrate a significant difference in anterior tibial translation between the operated and non-operated knees. Overall, 53 patients were recruited to allow for attrition over the assessment period.
For all subjective (PROMs) and objective outcomes, the means (SD, range) were presented at the designated assessment time-points, whilst repeated-measures analysis of variance (ANOVA) was employed to assess change in these outcomes over time. Limb Symmetry Indices (LSIs) were calculated and presented for the hop and strength tests, further categorized by the number and percentage of patients with LSIs ≥ 90% for all four hop tests (at each time-point), as well as all hop tests combined with peak isokinetic knee extension and flexion torque. For KT-1000 anterior tibial translation measures, t tests were employed to compare the operated and non-operated limbs at 6 months post-surgery, whilst repeated-measures ANOVA assessed any change in the side-side limb anterior tibial translation difference over time. KT-1000 anterior tibial translation measures were further categorized based on side-to-side difference as normal (< 3 mm), nearly normal (3–5 mm), abnormal (6–10 mm) and severely abnormal (> 10 mm) [39]. The NSARS was employed to present the number (and percentage) of patients participating in Level 1 (participation 4–7 days/week) or Level 2 (participation 1–3 days per week) activities that included jumping, hard pivoting and cutting sports pre-injury and at 12- and 24 months post-surgery. The number (and percentage) of patients reporting ‘Very Satisfied’, ‘Somewhat Satisfied’, ‘Somewhat Dissatisfied’ and ‘Very Dissatisfied’ within each of the satisfaction domains at 24 months post-surgery was presented. The number (and type) of surgical complications, adverse events, re-operations and re-injuries were presented. Where appropriate, statistical analysis was performed using SPSS software (SPSS, Version 27.0, SPSS Inc., USA), with statistical significance determined at p < 0.05.
Results
Patient demographics and injury/surgery parameters of the 53 patients that were recruited and underwent surgery are demonstrated in Table 2.
Objective results
With respect to the 4-month knee laxity exam undertaken by the senior author, all patients presented with a normal (or near normal) pivot shift clinical examination, with no Grade II or III pivot laxity outcomes. For the later-stage KT-1000 assessments, there were no significant anterior tibial translation differences between the operated and non-operated knees at 6 months post-surgery (p = 0.433), with no significant increase (p = 0.841) in side-to-side anterior tibial translation from 6 to 24 months (Table 3). At 24 months, KT-1000 measurements demonstrated normal (< 3 mm) or near normal (3–5 mm) side-to-side differences in 98.0% of patients (Table 3). Knee flexion and extension ROM significantly improved (p < 0.0001) over time, as did the LSI for peak isokinetic knee extensor torque (p < 0.0001), the SHD (p = 0.001), THD (p = 0.001) and TCHD (p < 0.0001) (Table 4). At 12 months post-surgery, 72.3% of patients presented with an LSI ≥ 90% for every hop test, which dropped to 53.2% of patients when combined with LSIs ≥ 90% for peak isokinetic knee extensor and flexor strength (Table 5). This was 79.6% of patients (all four hops) and 61.2% of patients (all four hops combined with strength measures) at 24 months post-surgery (Table 5).
Subjective results and return to sport
All PROMs significantly improved over time (p < 0.0001) (Table 6). As per the NSARS, 90.6% of patients were actively participating in Level 1 or 2 sports that included jumping, hard pivoting, cutting, running, twisting and/or turning pre-injury, which was 70.2% and 85.7% at 12 and 24 months post-surgery, respectively (Table 7). At 24-month review, 98.0% of patients were satisfied overall with their surgical outcome, with 93.9% satisfied with their ability to participate in sport (Table 8).
Complications, re-injuries and secondary surgical procedures
Over the course of the 24-month follow-up period, one patient presented with an early wound infection that was treated accordingly without further issue. Three patients underwent secondary surgical procedures, including one patient that underwent arthroscopic lateral meniscectomy for recurrent symptoms at 18 months after his primary ACLR (with an intact ACL at time of secondary surgery) and one patient that underwent lateral meniscal repair at 10 months after his primary ACLR (with an intact ACL at time of secondary surgery, albeit the meniscal tear was new and following a secondary incident). The third patient underwent medial meniscectomy at 6 months after his primary ACLR for recurrent symptoms and, whilst he was doing well and had returned to pivoting sports by 12 months, experienced an ACL re-tear at 17 months after his primary ACLR which continues to be managed non-operatively. This patient had a graft diameter of 9 mm. There were no further ipsilateral re-tears or contralateral tears. The data collected from these patients were still included in the results analysis.
Discussion
The most important finding from the current study was that an ACLR technique using autologous hamstrings augmented with a suture tape, combined with a structured post-operative rehabilitation programme, produced high-scoring PROMs and patient satisfaction with encouraging performance scores and RTS rates, without evidence of excessive anterior tibial translation and/or a high re-injury rate.
No difference in anterior tibial translation between the operated and non-operated limbs was observed, with 98% of patients demonstrating normal (< 3 mm) or near normal (3–5 mm) side-to-side differences up until 24 months post-surgery (the only patient who demonstrated side-to-side anterior tibial translation > 5 mm had suffered a known re-tear). This was in support of the first hypothesis. Further to this, as reported recently by Fiil et al. [40], excessive post-operative anterior tibial translation may be associated with worse knee-related quality of life, reduced function in sports and an increased revision rate. Whilst the rationale for graft augmentation is largely focussed on early graft reinforcement [9, 14], the true nature of this reinforcement capacity remains unknown, given the relative lack of biomechanical research on suture tape augmentation. A biomechanical study published by Massey et al. [41] reported a higher load to failure, stiffness and energy to failure when augmenting a graft with internal brace, though this was in the context of ACL repair (not reconstruction). In the current study, only one patient (2%) suffered an ACL re-injury with no contralateral ACL tears up until 24 months, also in support of the second hypothesis. However, it should be acknowledged that whilst Grindem et al. [6] reported an increased re-tear rate up until 9 months post-surgery after which time no further reduction in re-tear risk was observed, theoretically an elevated re-tear risk may extend well after the patient’s RTS so ongoing review is required. Whilst excessive synovitis and high failure rates had limited the ongoing early use of synthetics in ACLR [18,19,20,21,22,23,24,25], these complications were not observed in the current study.
In the current study, 70.2% of patients were actively participating in pivoting sports at 12 months post-surgery, which had increased to 85.7% at 24 months (noting that 90.6% of patients were actively participating in pivoting sports pre-injury). This supported the third hypothesis and, of further interest, the 24-month post-operative mean TAS was actually higher than the pre-injury TAS. Whilst similar RTS rates were previously reported in patients following ACLR augmented with LARS [13], Ardern et al. [2] reported that only 65% of patients return to their pre-injury level of sport, with 55% returning to competitive sport. The higher RTS rates may be influenced by a range of factors including participation and ongoing progression of rehabilitation, which was well adhered to in the current study. Further to this, the underlying rationale for the use of ACLR augmentation is that it may permit early ACL reinforcement and graft stability prior to graft incorporation, also accelerating rehabilitation [9, 14]. Of importance, the encouraging RTS rates currently observed did not appear to increase the risk of excessive anterior tibial translation or re-injury risk. It should be reiterated again that RTS was not advised until ≥ 9 months post-surgery and patients were counselled on specific objective criteria that should be ideally attained before RTS, though this could not be enforced and was at the final discretion of the patient.
High-scoring PROMs and high levels of patient satisfaction were reported, whilst mean LSIs ≥ 90% were reported at all post-operative time-points for peak isokinetic knee flexor strength and all hop measures. Furthermore, the mean LSI for peak isokinetic knee extensor strength was ≥ 90% at 12 and 24 months, albeit 75% and 82% at 6 and 9 months, respectively. This was largely in support of the fourth hypothesis. However, when grouped in the form of a performance test battery, 72% and 80% of patients presented with an LSI ≥ 90% for every hop test at 12 and 24 months, respectively. When this test battery further included LSIs ≥ 90% for the knee extensor and flexor strength measures, this was only 53% and 61% at 12 and 24 months, respectively. Despite the low re-injury rate currently observed, existing research has reported an increased re-injury risk if patients fail to meet LSIs ≥ 90% across a range of tests including strength and hop performance measures [6, 7]. In contrast, other research has suggested an increased risk of contralateral ACL injury in the presence of improved strength and/or hop performance symmetry [42, 43]. Therefore, the limitations of employing LSIs to present performance outcomes should be acknowledged, such as the variation in LSI ‘cut-off’ values employed [6, 44,45,46,47] and the potential for LSIs to overestimate function [48].
Whilst the current subjective, objective and RTS outcomes appear similar to those reported previously in patients undergoing ACLR augmented with LARS [13], and more recent longer term follow-ups of reconstruction/repair with and without other ligament augmentation devices have reported sound clinical results [49, 50], limited published outcomes exist presenting outcomes specifically after ACLR augmented with FiberTape®. Bodendorfer et al. [14] presented a retrospective comparison of outcomes in patients undergoing ACLR with and without FiberTape® suture augmentation, with augmentation demonstrating less pain, improved PROMs and improved early return to activity, without evidence of over-constraint. A retrospective cohort study published by Barnas et al. [51] reported comparable functional outcomes in patients undergoing surgery for partial ACL tears with synthetic augmentation using either a polyethylene terephthalate tape (Neoligaments) or FiberTape® suture augmentation. A recent retrospective comparison published by Hopper et al. [52] reported comparable re-injury and secondary surgery rates in patients undergoing ACLR versus those undergoing ACL repair with suture tape augmentation, in the context of acute proximal ACL ruptures. Finally, a recent systematic review published by Zheng et al. [53] specifically on the use of suture augmentation for ACLR reported overall favourable clinical outcomes and, whilst being associated with better sports performance compared to standard ACLR, was comparable in most functional scores, knee stability measures and graft failure rates. Most other ACLR papers employing FiberTape® augmentation are technical notes without patient outcomes [8, 12, 26]. A prospective 2-year study published by Heusdens et al. [27] reported improved post-operative outcomes of suture augmentation in the context of ACL repair, with a 4.8% re-rupture rate over the period, but other published papers using FiberTape® augmentation for ACL repair are also limited to technical notes [28, 29].
A number of limitations are acknowledged within the current study. First, it was a single centre study in patients undergoing a specific augmented ACLR technique that does not permit generalization. Furthermore, we acknowledge that there was no comparative group with the current study and, based on the early clinical experience our group had with this augmented ACLR technique, our initial plan was to undertake a robust prospective evaluation of patients undergoing this ACLR technique with close and frequent assessment of outcomes and adverse events, with comparison to existing literature where appropriate. This now provides a framework for a subsequent randomized comparative study. Additionally, it may be argued that it was a heterogeneous group with a wide age range (16–45 years) and almost 50% of patients undergoing concomitant meniscal surgery, though this is also a strength in presenting outcomes in a common community-level cohort embarking on ACLR. Second, we acknowledge that the primary study aim and sample size calculation was focussed around excessive anterior tibial translation (KT-1000 measurements), and both the 4-month pivot shift clinical review, as well as the 6-, 9-, 12- and 24-month KT-1000 reviews, were undertaken on the patient (on both limbs for the KT-1000) in an awake condition, which may be less reliable than an anaesthetized environment. Third, whilst an aim was to report on RTS rates at 12 and 24 months, the actual time to RTS was not documented. Finally, whilst it is acknowledged that rehabilitation can affect strength and function after ACLR [45, 54, 55] and patients underwent a structured rehabilitation programme following surgery (also seeking to document rehabilitation adherence), it is acknowledged that in many community-level ACLR patients, rehabilitation will differ, as will individual patient motivation and exercise diligence.
Conclusion
The current study has demonstrated that ACLR using autologous hamstrings augmented with the suture tape, combined with a structured, post-operative rehabilitation programme, produced high-scoring PROMs and patient satisfaction with encouraging performance scores and RTS rates, without evidence of excessive anterior tibial translation and/or a high re-injury rate. Particularly given the high RTS rates at 24 months post-surgery, ongoing patient review is required to further investigate latter stage re-injury rates.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Zbrojkiewicz D, Vertullo C, Grayson JE (2018) Increasing rates of anterior cruciate ligament reconstruction in young Australians, 2000–2015. Med J Aust 208(8):354–8. https://www.ncbi.nlm.nih.gov/pubmed/29669497
Ardern CL, Taylor NF, Feller JA, Webster KE (2014) Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med 48(21):1543–1552. https://doi.org/10.1136/bjsports-2013-093398
Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD (2016) Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med 44(7):1861–1876. https://doi.org/10.1177/0363546515621554
Samitier G, Marcano AI, Alentorn-Geli E, Cugat R, Farmer KW, Moser MW (2015) Failure of anterior cruciate ligament reconstruction. Arch Bone Jt Surg 3(4):220–40. https://www.ncbi.nlm.nih.gov/pubmed/26550585.
Hayback G, Raas C, Rosenberger R (2022) Failure rates of common grafts used in ACL reconstructions: a systematic review of studies published in the last decade. Arch Orthop Trauma Surg 142(11):3293–3299. https://doi.org/10.1007/s00402-021-04147-w
Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA (2016) Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware–Oslo ACL cohort study. Br J Sports Med 50(13):804–808. https://doi.org/10.1136/bjsports-2016-096031
Kyritsis P, Bahr R, Landreau P, Miladi R, Witvrouw E (2016) Likelihood of ACL graft rupture: not meeting six clinical discharge criteria before return to sport is associated with a four times greater risk of rupture. Br J Sports Med 50(15):946–951. https://doi.org/10.1136/bjsports-2015-095908
Saper MG (2018) Quadriceps tendon autograft anterior cruciate ligament reconstruction with independent suture tape reinforcement. Arthrosc Tech 7(11):e1221–e1229. https://doi.org/10.1016/j.eats.2018.08.007
Falconer TM, Tusak L, Breidahl WH, Annear PT (2015) The LARS augmented 4-tunnel hamstring “hybrid” ACLR graft construction allows accelerated rehabilitation without knee laxity—case series of 111 patients after 2 years. J Musc Res. https://doi.org/10.1142/S0218957715500207
Hamido F, Al Harran H, Al Misfer AR, El Khadrawe T, Morsy MG, Talaat A et al (2015) Augmented short undersized hamstring tendon graft with LARS(R) artificial ligament versus four-strand hamstring tendon in anterior cruciate ligament reconstruction: preliminary results. Orthop Traumatol Surg Res 101(5):535–538. https://doi.org/10.1016/j.otsr.2015.01.021
Hamido F, Misfer AK, Al Harran H, Khadrawe TA, Soliman A, Talaat A et al (2011) The use of the LARS artificial ligament to augment a short or undersized ACL hamstrings tendon graft. Knee 18(6):373–378. https://doi.org/10.1016/j.knee.2010.09.003
Smith PA, Bley JA (2016) Allograft anterior cruciate ligament reconstruction utilizing internal brace augmentation. Arthrosc Tech 5(5):e1143–e1147. https://doi.org/10.1016/j.eats.2016.06.007
Ebert JR, Annear PT (2019) ACL reconstruction using autologous hamstrings augmented with the ligament augmentation and reconstruction system provides good clinical scores, high levels of satisfaction and return to sport, and a low retear rate at 2 years. Orthop J Sports Med 7(10):2325967119879079. https://doi.org/10.1177/2325967119879079
Bodendorfer BM, Michaelson EM, Shu HT, Apseloff NA, Spratt JD, Nolton EC et al (2019) Suture augmented versus standard anterior cruciate ligament reconstruction: a matched comparative analysis. Arthroscopy 35(7):2114–2122. https://doi.org/10.1016/j.arthro.2019.01.054
Batty LM, Norsworthy CJ, Lash NJ, Wasiak J, Richmond AK, Feller JA (2015) Synthetic devices for reconstructive surgery of the cruciate ligaments: a systematic review. Arthroscopy 31(5):957–968. https://doi.org/10.1016/j.arthro.2014.11.032
Aujla RS, Ebert JR, Annear PT (2021) Anterior cruciate ligament reconstruction using autologous hamstrings augmented with the ligament augmentation and reconstruction system versus hamstrings alone: a comparative cohort study. Orthop J Sports Med 9(10):23259671211046630. https://doi.org/10.1177/23259671211046631
Chen T, Zhang P, Chen J, Hua Y, Chen S (2017) Long-term outcomes of anterior cruciate ligament reconstruction using either synthetics with remnant preservation or hamstring autografts: a 10-year longitudinal study. Am J Sports Med 45(12):2739–2750. https://doi.org/10.1177/0363546517721692
Jenkins DH (1978) The repair of cruciate ligaments with flexible carbon fibre. A longer term study of the induction of new ligaments and of the fate of the implanted carbon. J Bone Joint Surg Br 60-B(4):520–2. https://www.ncbi.nlm.nih.gov/pubmed/711800
Kdolsky RK, Gibbons DF, Kwasny O, Schabus R, Plenk H Jr (1997) Braided polypropylene augmentation device in reconstructive surgery of the anterior cruciate ligament: long-term clinical performance of 594 patients and short-term arthroscopic results, failure analysis by scanning electron microscopy, and synovial histomorphology. J Orthop Res 15(1):1–10. https://doi.org/10.1002/jor.1100150102
Kumar K, Maffulli N (1999) The ligament augmentation device: an historical perspective. Arthroscopy 15(4):422–32. https://www.ncbi.nlm.nih.gov/pubmed/10355719
Makisalo SE, Visuri T, Viljanen A, Jokio P (1996) Reconstruction of the anterior cruciate ligament with carbon fibres: unsatisfactory results after 8 years. Knee Surg Sports Traumatol Arthrosc 4(3):132–6. https://www.ncbi.nlm.nih.gov/pubmed/8961226
Rading J, Peterson L (1995) Clinical experience with the Leeds–Keio artificial ligament in anterior cruciate ligament reconstruction. A prospective two-year follow-up study. Am J Sports Med 23(3):316–319. https://doi.org/10.1177/036354659502300311
Richmond JC, Manseau CJ, Patz R, McConville O (1992) Anterior cruciate reconstruction using a Dacron ligament prosthesis. A long-term study. Am J Sports Med 20(1):24–28. https://doi.org/10.1177/036354659202000107
Woods GW (1985) Synthetics in anterior cruciate ligament reconstruction: a review. Orthop Clin N Am 16(2):227–35. https://www.ncbi.nlm.nih.gov/pubmed/2987770
Zoltan DJ, Reinecke C, Indelicato PA (1988) Synthetic and allograft anterior cruciate ligament reconstruction. Clin Sports Med 7(4):773–84. https://www.ncbi.nlm.nih.gov/pubmed/3052882.
Lavender C, Johnson B, Kopiec A (2018) Augmentation of anterior cruciate ligament reconstruction with bone marrow concentrate and a suture tape. Arthrosc Tech 7(12):e1289–e1293. https://doi.org/10.1016/j.eats.2018.08.020
Heusdens CHW, Hopper GP, Dossche L, Roelant E, Mackay GM (2019) Anterior cruciate ligament repair with independent suture tape reinforcement: a case series with 2-year follow-up. Knee Surg Sports Traumatol Arthrosc 27(1):60–67. https://doi.org/10.1007/s00167-018-5239-1
Tapasvi SR, Shekhar A, Patil SS (2018) Primary anterior cruciate ligament repair with augmentation. Arthrosc Tech 7(2):e139–e145. https://doi.org/10.1016/j.eats.2017.08.063
van der List JP, DiFelice GS (2017) Arthroscopic primary anterior cruciate ligament repair with suture augmentation. Arthrosc Tech 6(5):e1529–e1534. https://doi.org/10.1016/j.eats.2017.06.009
Reid A, Birmingham TB, Stratford PW, Alcock GK, Giffin JR (2007) Hop testing provides a reliable and valid outcome measure during rehabilitation after anterior cruciate ligament reconstruction. Phys Ther 87(3):337–349. https://doi.org/10.2522/ptj.20060143
Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P et al (2001) Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med 29(5):600–13. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11573919
Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD (1998) Development of a patient-reported measure of function of the knee. J Bone Jt Surg Am 80(8):1132–45. http://www.ncbi.nlm.nih.gov/pubmed/9730122
Barber-Westin SD, Noyes FR, McCloskey JW (1999) Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med 27(4):402–16. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10424208
Lysholm J, Gillquist J (1982) Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med 10(3):150–4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6896798
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198(198):43–9. https://www.ncbi.nlm.nih.gov/pubmed/4028566
Webster KE, Feller JA, Lambros C (2008) Development and preliminary validation of a scale to measure the psychological impact of returning to sport following anterior cruciate ligament reconstruction surgery. Phys Ther Sport 9(1):9–15. https://doi.org/10.1016/j.ptsp.2007.09.003
Noyes FR, Barber SD, Mooar LA (1989) A rationale for assessing sports activity levels and limitations in knee disorders. Clin Orthop Relat Res (246):238–49. http://www.ncbi.nlm.nih.gov/pubmed/2670388
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Lawrence Erlbaum Associates, Hillsdale
Nicholas SJ, D'Amato MJ, Mullaney MJ, Tyler TF, Kolstad K, McHugh MP (2004) A prospectively randomized double-blind study on the effect of initial graft tension on knee stability after anterior cruciate ligament reconstruction. Am J Sports Med 32(8):1881–6. http://www.ncbi.nlm.nih.gov/pubmed/15572316
Fiil M, Nielsen TG, Lind M (2022) A high level of knee laxity after anterior cruciate ligament reconstruction results in high revision rates. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-022-06940-5
Massey P, Parker D, McClary K, Robinson J, Barton RS, Solitro GF (2020) Biomechanical comparison of anterior cruciate ligament repair with internal brace augmentation versus anterior cruciate ligament repair without augmentation. Clin Biomech (Bristol, Avon) 77:105065. https://doi.org/10.1016/j.clinbiomech.2020.105065
Cristiani R, Forssblad M, Edman G, Eriksson K, Stalman A (2022) Age, time from injury to surgery and hop performance after primary ACLR affect the risk of contralateral ACLR. Knee Surg Sports Traumatol Arthrosc 30(5):1828–1835. https://doi.org/10.1007/s00167-021-06759-6
Webster KE, Hewett TE (2019) What is the evidence for and validity of return-to-sport testing after anterior cruciate ligament reconstruction surgery? A systematic review and meta-analysis. Sports Med 49(6):917–929. https://doi.org/10.1007/s40279-019-01093-x
Keays SL, Bullock-Saxton JE, Newcombe P, Keays AC (2003) The relationship between knee strength and functional stability before and after anterior cruciate ligament reconstruction. J Orthop Res 21(2):231–237. https://doi.org/10.1016/S0736-0266(02)00160-2
Ebert JR, Edwards P, Yi L, Joss B, Ackland T, Carey-Smith R et al (2018) Strength and functional symmetry is associated with post-operative rehabilitation in patients following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 26(8):2353–2361. https://doi.org/10.1007/s00167-017-4712-6
Schmitt LC, Paterno MV, Hewett TE (2012) The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther 42(9):750–759. https://doi.org/10.2519/jospt.2012.4194
Barfod KW, Feller JA, Hartwig T, Devitt BM, Webster KE (2019) Knee extensor strength and hop test performance following anterior cruciate ligament reconstruction. Knee 26(1):149–154. https://doi.org/10.1016/j.knee.2018.11.004
Wellsandt E, Failla MJ, Snyder-Mackler L (2017) Limb symmetry indexes can overestimate knee function after anterior cruciate ligament injury. J Orthop Sports Phys Ther 47(5):334–338. https://doi.org/10.2519/jospt.2017.7285
Sporsheim AN, Gifstad T, Lundemo TO, Engebretsen L, Strand T, Molster A et al (2019) Autologous BPTB ACL reconstruction results in lower failure rates than ACL repair with and without synthetic augmentation at 30 years of follow-up: a prospective randomized study. J Bone Jt Surg Am 101(23):2074–2081. https://doi.org/10.2106/JBJS.19.00098
Elveos MM, Drogset JO, Engebretsen L, Bronn R, Lundemo TO, Gifstad T (2018) Anterior cruciate ligament reconstruction using a bone-patellar tendon-bone graft with and without a ligament augmentation device: a 25-year follow-up of a prospective randomized controlled trial. Orthop J Sports Med 6(11):2325967118808778. https://doi.org/10.1177/2325967118808778
Barnas M, Kentel M, Morasiewicz P, Witkowski J, Reichert P (2021) Clinical assessment and comparison of ACL reconstruction using synthetic graft (Neoligaments versus FiberTape). Adv Clin Exp Med 30(5):491–498. https://doi.org/10.17219/acem/132036
Hopper GP, Wilson WT, O’Donnell L, Hamilton C, Blyth MJG, MacKay GM (2022) Comparable rates of secondary surgery between anterior cruciate ligament repair with suture tape augmentation and anterior cruciate ligament reconstruction. J Exp Orthop 9(1):115. https://doi.org/10.1186/s40634-022-00549-w
Zheng T, Cao Y, Song G, Li Y, Zhang Z, Feng Z et al (2022) Suture tape augmentation, a novel application of synthetic materials in anterior cruciate ligament reconstruction: a systematic review. Front Bioeng Biotechnol 10:1065314. https://doi.org/10.3389/fbioe.2022.1065314
Failla MJ, Logerstedt DS, Grindem H, Axe MJ, Risberg MA, Engebretsen L et al (2016) Does extended preoperative rehabilitation influence outcomes 2 years after ACL reconstruction? A comparative effectiveness study between the MOON and Delaware–Oslo ACL cohorts. Am J Sports Med 44(10):2608–2614. https://doi.org/10.1177/0363546516652594
Grindem H, Granan LP, Risberg MA, Engebretsen L, Snyder-Mackler L, Eitzen I (2015) How does a combined preoperative and postoperative rehabilitation programme influence the outcome of ACL reconstruction 2 years after surgery? A comparison between patients in the Delaware–Oslo ACL Cohort and the Norwegian National Knee Ligament Registry. Br J Sports Med 49(6):385–389. https://doi.org/10.1136/bjsports-2014-093891
Acknowledgements
We acknowledge Arthrex for their research grant assistance.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. An independent research grant was provided by Arthrex (US-00010) to assist with this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No benefits in any form have been received or will be received relating to this article.
Ethics
Ethics approval was obtained by the University of Western Australia (RA/4/20/1046).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ebert, J.R., Edwards, P. & Annear, P.T. Good clinical scores, no evidence of excessive anterior tibial translation, a high return to sport rate and a low re-injury rate is observed following anterior cruciate ligament reconstruction using autologous hamstrings augmented with suture tape. Arch Orthop Trauma Surg 143, 5207–5220 (2023). https://doi.org/10.1007/s00402-023-04835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-04835-9