Abstract
Introduction
After conventional surgical refixation of the hamstrings after proximal hamstring rupture, patients frequently experience pain while sitting and deficits in hamstring muscle strength of the operated side. To improve these outcomes, we have modified the surgical anchor placement and have carried out a thorough follow-up examination.
Materials and methods
Thirteen older patients (8 female, 5 males) with a median age of 64.2 (range, 52.1–80.4) years were surgically treated for acute proximal hamstring rupture using modified anchor placement and participated in a follow-up assessment at a median of 46.2 (11.2–75.0) months after surgery. Patients completed the Perth Hamstring Assessment Tool (PHAT), quality of life questionnaire (EQ-5D-5L) and the Lower Extremity Functional Scale (LEFS), and rated their satisfaction level on a scale from 0 to 100%. Local tenderness on the ischial tuberosity and maximum passive hip flexion were measured on both limbs. Maximum isokinetic knee flexor muscle strength was measured bilaterally using a dynamometer.
Results
The median (range) PHAT, EQ-5D-5L and LEFS score were 78.8/100 (54.6–99.8), 0.94/1 (0.83–1) and 88.75/100 (61.25–100). The median satisfaction was 100% (90–100%). Only one patient felt discomfort when the ischial tuberosity was palpated. Neither maximum passive hip flexion nor maximum isokinetic flexor muscle strength differed between the operated and non-operated side (P > 0.58). Clinical scores did not correlate with the leg symmetry index of knee flexor muscle strength (Spearman’s rho < 0.448, P > 0.125). There were no tendon re-ruptures, or postoperative sciatic radiculopathy, at the time of follow-up.
Conclusions
The modified extra-anatomical anchor placement resulted in good clinical and functional outcome of surgical repair of acute proximal hamstring rupture. Especially the absence of postoperative pain while sitting and the comparable muscle strength to the contralateral side is promising.
Clinical trial registration
ClinicalTrials.gov Identifier: NCT04867746, registered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proximal hamstring rupture can affect trained athletes as well as the general population. For instance, the prevalence of proximal hamstring rupture in sports such as soccer, American football, baseball or track and field ranges from 5 to 40% and may depend on hours of sport exposure [1,2,3,4,5]. The most common injury mechanism is a simultaneous knee extension with hip flexion [6]. In adults, the conjoint tendon is most commonly involved in the rupture with or without a combination with the semimembranosus muscle [7]. There is a general consensus outlined in reviews that in case of complete rupture of all three tendons (i.e., semitendinosus, semimembranosus and biceps femoris muscles) or retraction (i.e., shortening) of at least two tendons by more than 2 cm, surgical treatment has a superior outcome than conservative treatment [6, 8,9,10,11,12,13] although there is no literature on the conservative management of older people with hamstring ruptures. Moreover, the current evidence suggests that the injured muscle should be reinserted on the original anatomical footprint in case of surgical refixation thereby closely restoring the natural anatomy [14,15,16,17]. The anatomical origin of the hamstring muscles on the ischial tuberosity is well known [18]. The semimembranosus muscle attachment is located more proximal and lateral than the attachment site of the conjoint tendon of the long head of the biceps femoris muscle and the semitendinosus muscle [13, 18, 19].
Some undesirable postoperative functional and clinical outcomes have been reported. Postoperative pain when seated has been frequently reported as the most common postoperative complaint and is seen in up to 61% of cases [19] with a mean pain score while seated of 64 (visual analogue scale, range 0–100) [20]. The ischial tuberosity—the anatomical origin of the hamstring muscles—is the point of maximal pressure and force transfer from the upper body on the seat surface while sitting [19, 21, 22] resulting, for instance, in irritation and/or pain in athletes after a first longer bike ride at the beginning of a cycling season [23]. Patients after anatomical proximal hamstring refixation onto the ischial tuberosity may experience pain due to the implant positioning, scar tissue or thickening of the tendon at the origin. Because people in developed countries spend more and more time sitting [24], addressing the complaint of pain while sitting in patients after proximal hamstring rupture is critical.
In addition, a decrease in maximum strength of the knee flexors has been described and accepted as an inevitable outcome [25]. This reported knee flexor strength deficit ranges from 12 to 15% [13, 26] to of up to 26% [16]. However, the existing literature on hamstring repair includes younger patients and/or athletes [13], and evidence for the outcome of hamstring repair in older patients who are not athletically ambitious are largely lacking. Because the capacity to produce force depends on the length of a muscle, proper muscle tensioning during surgical muscle repair is important. The force–length relationship of a muscle [27] implies that slight pre-tensioning of the muscle—achieved by minor stretching—can optimize its power output [27,28,29,30]. Hence, by pre-tensioning the injured muscle the surgeon can influence postoperative force capacity of the repaired muscle and hence its maximum muscle strength capacity as well as the muscle length at which this strength occurs.
Based on these considerations, we aimed to improve the current surgical technique by
-
refixing the hamstring tendons proximal and lateral of their anatomical origin to pre-tensioning the muscle and hence.
-
moving the insertion site of the tendons—especially of the conjoint tendon—away from the region loaded while sitting.
Combining these two modifications results in a modified extraanatomical insertion point on the supero-lateral side of the ischial tuberosity (Fig. 1). The objective of this study was to describe this modified surgical technique, to report clinical and functional outcome and patient satisfaction 1 to 6 years after proximal hamstring muscle repair with modified surgical anchor placement and to determine the association among these parameters in older patients with acute hamstring tendon rupture. We hypothesized that the modified anchor placement will result in good clinical outcome (low pain when seated, no palpation tenderness, no complications, high patient satisfaction), that the range of motion of the passive hip flexion, isokinetic muscle strength and thigh girth in the injured leg are comparable to those in the contralateral uninjured side and that clinical scores correlate with side-to-side differences in isokinetic muscle strength.
Illustration of the modified anchor placement. A anatomical attachment of proximal hamstring tendon on the ischial tuberosity of the right hip (1, yellow area); B modified, 10 to 15 mm (depending on the patients’ anatomy) more proximal and lateral attachment of both tendons (2, green area). Note that this anchor placement does not involve anchor placement on the caudal portion of ischial tuberosity
Methods
Overall, 17 older patients were surgically treated within 4 weeks after acute proximal hamstring rupture between 2014 and 2019 at a single hospital by a single senior surgeon and all received the extraanatomical anchor positioning procedure. All patients were contacted retrospectively at the time of the study (November 2020 to June 2021) by the administrative personnel of our clinic and asked to participate in the follow-up examination. Inclusion criteria were age > 18 years and surgical repair with modified anchor placement surgically treated proximal hamstring ruptures between 2014 and 2019. Exclusion criteria were revision surgery within 6 months before testing on the ipsilateral knee and hip, injury and surgical procedures of the contralateral knee and hip within the last year, and inability to provide informed consent. The study was approved by the regional ethics committee. All participants signed informed consent prior to participation.
Of the 17 patients treated, 13 agreed to participate. One patient was not interested in the follow-up assessment because he was highly satisfied with the result of the operation. One patient only locomoted with an electric wheelchair, had just suffered a COVID-19 infection and was medically incapacitated to participate at the follow-up examination. One patient had severe acute rheumatic disease and one patient could not be reached via phone, email, or postal service. Two patients were only able to participate at specific dates because of personal and professional obligations and hence were allowed to participate before reaching the 24-month follow-up time (11 and 21 months after surgery, respectively).
Surgical technique
An intubation or spinal anaesthesia was used, and a preoperative single shot of antibiotics with Cefazolin 2 g was intravenously administered according to the hospital standards. The patient was positioned prone with 60° hip flexion and simultaneous 60° knee flexion. Special care was taken to carefully support the patient’s body by padded surgical cushions to prevent a build-up of decubitus. A sterile gaze was precisely taped to fully cover the rima ani. A self-adhesive, sterile drape was then glued around the ipsilateral gluteus and the posterior part of the proximal thigh, to allow sufficient surgical exposure. The lower leg was fully covered with sterile stockinet and taped shut. The entire surgical aperture was then covered with an antimicrobial Ioban foil (3 M™ Ioban™ Steri-Drape™, 3 M™, MN, USA). The skin incision was performed strictly in the gluteal sulcus. Under a constant haemostasis with bipolar forceps, the incision was deepened to the deep fascia, which was displayed and horizontally incised. The caudal border of musculus gluteus maximus was identified and moved proximally. Medially and caudally from it, the hamstring fascia was vertically incised. The exposure was held in place with Langenbeck retractors and medially as well as laterally around ischial tuberosity placed Hohmann retractors. The sciatic nerve was not specifically searched for but great care was taken not to damage or compress it when placing the retractors.
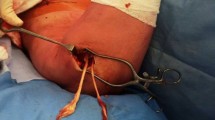
The torn proximal hamstring tendons were identified, and a surgical debridement was performed. Subsequently, the hamstring origin on the ischial tuberosity was localized. We projected the modified reinsertion point 10 to 15 mm (depending on the patients’ anatomy) more lateral and proximal of the ischial tuberosity. This location was thoroughly debrided using a Luer forceps and three Arthrex BioComposite Corkscrews (FT 5.5 mm × 14.7 mm anchors, Arthrex, FA, USA) were introduced into the bone. Two of these were placed proximally and one distally. The non-resorbable FibreWire anchor-threads (FibreWire, Arthrex, FA, USA) were tightly sutured through the full thickness of the hamstring tendon using a Baseball-Stitch-Technique from proximal to distal. The armed tendon was then compressed using a Pulley-Method against the debrided bone and the anchors. After tying the surgical square knots, the remaining FibreWire was cut away (Fig. 2). Then, the wound was irrigated multiple times with Ringer-Lactate and the haemostasis was completed. The deep fascia of gluteus maximus musculature was sutured. The closure of the skin was subcutaneously performed with single sutures and the dermis itself with an intradermal resorbable suture and steri-strips. The wound was then covered with watertight plaster.
Intraoperative photographs. A torn proximal hamstring tendon is held with Kocher forceps; B debrided proximal and lateral part of ischial tuberosity just before anchor placement; C modified anchor placement with two anchors proximally and one distally; D completely refixed and adapted hamstring tendon. For illustrative purposes, we have added pelvic bone projections
Postoperatively, the patients were allowed to partially bare weight (15 kg) and were constantly wearing a knee brace (Hinged Knee Brace, DonJoy, TX, USA) allowing knee extension up to 30° knee flexion angle for 8 weeks after the surgery. Hip flexion was not mechanically limited, but the patients were advised not to flex the hip more than 60° for 8 weeks after the surgery. First postoperative mobilisation took place under the control of a physiotherapist, and the patients were discharged when mobilisation and walking stairs with crutches was performed independently and safely. The supervised physiotherapy continued after hospital discharge. Initially, only procedures for muscle relaxation were allowed. After 4 weeks, isometric muscle strengthening and patellar mobilisation was introduced. Stretching of the hamstrings was prohibited for 4 months postoperatively. A daily use of thromboembolic prophylaxis with Deltaparin natrium (M.R. 4000–6000 D) (5000 UI s.c.) was prescribed for 8 weeks. Stitch removal was not necessary because of the use of a resorbable intradermal skin suture. Clinical follow-up was performed at 6 to 8 weeks postoperatively.
Clinical and functional outcome assessment
At the single follow-up study visit, all patients completed the Perth Hamstring Assessment Tool (PHAT) [14, 31], a quality of life questionnaire (EQ-5D-5L) [32] and the Lower Extremity Functional Scale (LEFS) [33]. The PHAT assessment tool is a quick and reliable tool for measuring and comparing interindividual results in patients after a surgical treatment of a proximal hamstring rupture [31]. Additional to the over-all PHAT score, two of the PHAT-subscores (visual analogue scale (VAS): VAS when sitting, VAS at rest; 0–no pain; 100–maximum pain) were also included in further analyses. The EQ-5D-5L and LEFS scores are widely used cost-effective and simple methods for assessing the subjective perception, impairment and functionality of lower extremity in daily life after surgery [32, 33]. Additionally, patients were asked to rate their subjective satisfaction with the outcome of the surgery on a scale from 0 to 100% (0%–not satisfied at all; 100%–extremely satisfied).
A physical examination of both limbs of the patients was performed by a senior orthopaedic resident. Patients were asked to lie in prone position on a flat examination table. To assess local tenderness, the ischial tuberosity was palpated, and patients were asked whether they felt pain (yes/no) [34]. Patients were asked to turn to a supine position, and maximum passive hip flexion of both hip joints was examined [34,35,36]. Two measurements were made for each limb: maximum hip flexion with simultaneously flexed knee and maximum hip flexion with knee in full extension [9]. Hip flexion angles were measured with a goniometer centred on the previously palpated lateral tip of the greater trochanter.
Maximum isokinetic muscle strength of the knee flexors and extensors was measured for both limbs using an isokinetic dynamometer (Biodex Medical Systems 4 Pro, Mirion Technologies, GA, USA) by a trained movement scientist. The reliability, validity and accuracy of this system has been previously reported [37]. Patients were seated on the dynamometer, asked to freely test and familiarize with the knee movement permitted by the dynamometer. Individual maximal knee flexion and extension were set and registered on the dynamometer. Then, patients were asked to perform a series of five knee flexion and extension cycles at a velocity of 60°/s [37] using the previously determined maximum range of motion. After a break of 30 s, a second series of five cycles was executed. The maximum knee flexion torque of both series was used for each side, normalized to body mass (Nm/kg), and used for further analysis. Thigh girth was measured for both limbs 10 cm proximal of base of the patella using a tape measure.
Statistical analysis
All study related data were collected and managed using REDCap electronic data capture tools hosted at our institution [38, 39]. All analyses were performed in SPSS Version 25 (IBM Corporation, Amonk, NY, USA). Significant differences in range of motion, muscle strength and thigh girth between the affected and the contralateral side were detected using Wilcoxon signed ranks tests. The limb symmetry index (LSI) was calculated as the maximum muscle flexion torque of the affected limb divided by the value for the unaffected limb multiplied by 100. An LSI of 90–110% can be considered as normal [40]. Spearman’s rank correlation coefficients were used to detect a potential correlation between the clinical scores and the LSI.
Results
Of the 13 participants, 8 were females and 5 were males. The median age at follow-up was 64.2 years (range 52.1–80.4 years). The median time between trauma and surgery was 14 days. At follow-up, participants had a median body mass index of 28.5 kg/m2 (range 23.5–45 kg/m2). In seven patients the right side was injured, and in six patients the left side was injured. Twelve patients had a total (semitendinosus and biceps conjoint tendon and semimembranosus tendon) and one patient a partial (semitendinosus and biceps conjoint tendon) proximal hamstring rupture. The median time to follow-up assessment was 46.2 months (range 11.2–75.1 months) after surgery.
PHAT, EQ-5D-5L, LEFS, subjective satisfaction and local tenderness
Median scores of clinical questionnaires (PHAT, EQ-5D-5L, LEFS) are shown in Table 1. All patients were very to extremely satisfied with the result of the surgery with a median subjective satisfaction rate of 100% (range 90–100%). Only one patient indicated local tenderness during palpation of the ischial tuberosity of the injured side (incidence of 7.7%). None of the patients experienced local tenderness on the contralateral side.
Range of motion
Patients had a median maximum passive hip flexion with the knee in flexion of 120° (range 115–140°) on the operated side and 120° (range 115–150°) on the contralateral side (P = 0.581; Fig. 3). Patients had a median maximum passive hip flexion with the knee in extension of 90° (range 60–120°) on the operated side and 90° (range 70–110°) on the contralateral side (P = 1.000). Patients with greater values on the operated side had greater values on the contralateral side (Fig. 3).
Box plots showing the median and interquartile range of the maximum passive hip flexion with the knee in flexion (top left) and maximum passive hip flexion with the knee in extension (top right) of the injured and contralateral leg; scatter plots showing the relationship between maximum hip flexion of the injured and contralateral leg with knee in flexion (bottom left) and knee in extension (bottom right)
Isokinetic muscle strength
Patients had a median maximum flexor muscle strength of 0.82 Nm/kg (range 0.33–1.42 Nm/kg) on the operated side and 0.72 Nm/kg (range 0.41–1.89 Nm/kg) on the contralateral side (P = 0.807; Fig. 3). The median LSI of maximum flexor muscle strength was 95.6% (range 71.7–135.6%) corresponding to a median muscle strength deficit of 3.5% in the operated side. Thigh girth did not differ between the operated and the contralateral leg (operated: median 48.5 cm, range 43–63.5 cm; contralateral: median 48.5 cm, range 44.5–62.0 cm; P = 1.000). Patients with greater muscle strength and thigh girth on the operated side had greater values on the contralateral side (Figs. 4 and 5).
Box plots with median and interquartile range of the isokinetic knee extension torques of the injured and contralateral leg (left) and isokinetic knee flexion torques of the injured and contralateral leg (middle) and scatter plot showing the relationship between knee flexion muscle strength of the injured and contralateral leg (right)
Association between clinical scores and LSI
We found no significant correlations between any of the clinical scores (PHAT, EQ-5D, or LEFS) and the LSI in flexor muscle strength (Spearman’s rho < 0.448, P > 0.125; Table 1).
Postoperative complications
One patient had a superficial wound infection, which was successfully surgically treated and healed without any further problems. One patient had local skin-dehiscence, which was excised and newly sutured. There were no tendon re-ruptures and no postoperative sciatic radiculopathy at the time of follow-up.
Discussion
The objective of this study was to describe a modified surgical technique, to report clinical and functional outcome and patient satisfaction around 2 to 5 years after acute proximal hamstring muscle repair with modified surgical anchor placement, and to determine the association among these parameters in older patients. Our results confirmed our hypothesis that isokinetic muscle strength and thigh girth in the injured leg are comparable to those in the contralateral uninjured side. Contrary to our secondary hypothesis, clinical scores did not correlate with side-to-side differences in isokinetic muscle strength assessed as LSI of maximum knee flexion torque. Moreover, patient satisfaction was very high, and we observed few complications. These results suggest that the modified surgical anchor placement is a promising technique with good to excellent clinical and functional outcome.
The subjective satisfaction rate in our patients was extremely high and comparable to non-modified hamstring refixation (98.8 vs. 97% [19]). Similarly, the PHAT score was comparable to what was reported for patients after other surgical refixation methods (78.8 vs. 74.1 [41], 79.8 [42] and 79.9 [43]). The questions with the lowest points in the PHAT were number of minutes without discomfort while driving a car, number of minutes without discomfort while running and description of the current level of activity. The patients in our study were older patients with an age range of 52–80 years. One older female patient did not own or drive a car so she marked the answer “zero minutes without discomfort while driving a car”. Similarly, some patients did not participate in any sports and also did not run, and hence marked “zero minutes without discomfort while running” and that they were not able to play sports. These results suggest that this questionnaire may not be well suited for a generally less active population.
The importance of reducing the high incidence of feeling pain while sitting [19, 44] will become even more critical because the Western population spends increasing times sitting [24]. Moreover, chronic pain is the second most frequent reason for disability to work [45], and return-to-work programs are challenging [46, 47]. Most patients with proximal hamstring rupture are athletes and/or middle-aged persons [25] with a reported age around 42 to 47 years [19, 20, 44]. Hence, any surgical treatment should be designed to ensure about 20 more years of productive occupational work, which will usually be mostly in seated postures. In our study, only one of thirteen patients reported pain while sitting and local tenderness of ischial tuberosity corresponding to an incidence of 7.7%, which is markedly lower than the incidence of up to 61% incidence [25] reported in the literature. The treatment of proximal hamstring rupture has changed and progressed rapidly over the past few years. A systematic review reported superior outcome of surgical versus conservative therapy although evidence for conservative therapy is low and no study provided a direct comparison [13]. Moreover, consensus has been established that the likelihood of good postoperative result will increase with reducing the time from injury to the operation [26, 48]. These results emphasize the potential of further developing surgical techniques to achieve better clinical outcome as observed in our study.
We observed no measurable difference in maximum passive hip flexion between the operated and the contralateral hip, both with the knee in flexion as well as with the knee in extension. We also did not observe a difference in postoperative isokinetic maximum muscle strength between the operated and contralateral hamstring muscles. We noted two outliers in our measurements. One patient had an LSI of 135.6% corresponding to a 35.6% greater maximum flexor muscle strength on the operated side than on the contralateral side. Interestingly, this patient did not participate in any prescribed physiotherapy sessions but is a physiotherapist by training. We speculate that based on her background she was well aware of the importance of strength exercises and may have completed more rigorous training for a longer period than other patients. However, because we did not record or monitor completed physiotherapy sessions in this retrospective cross-sectional study, this remains speculation. Nonetheless, these results clearly show that hamstring muscle strength after this modified surgery may not only be comparable to but may far exceed the hamstring muscle strength of the contralateral side.
The other outlier had an LSI of 71.7%, corresponding to a 28.3% lower maximum flexor muscle strength on the operated side than on the contralateral side. This result was particularly surprising because this patient was still a semi-professional track and field athlete at the time of the follow-up visit. This patient had the highest maximum flexor muscle strength with noticeably weaker hamstring muscles on the operated compared to the contralateral side. This finding was surprising also to the patient because he had not experienced any functional limitations, had fully returned to sport, and was extremely satisfied with the result of the surgery.
In contrast to our study, reduced hamstring muscle strength after hamstring repair is still a common outcome. In some studies (all including younger cohorts than ours), patients were asked to subjectively estimate their hamstring muscle strength after a proximal hamstring refixation. The results showed a subjectively estimated residual strength of more or equal to 75% [19, 44], which must be considered unsatisfactory. In other words, the patients may subjectively estimate a muscle strength deficit of up to 25%, even though the previously reported objectively measured strength deficit may be only around 15% at 12 months after surgery [13, 26, 49]. This discrepancy emphasizes the need for an objective, quantitative, and, most importantly, comparable assessment of postoperative hamstring muscle strength between studies, as recommended by Reza et al. [50] and Fouasson-Chailloux et al. [49]. The overall deficit of peak flexor strength in the affected leg achieved in our patients (3.5%) is well below the 15% reported in the review by Fouasson-Chailloux et al. [49].
The very positive outcome of this modified surgical technique is likely linked to the modified positioning of the anchors. Overall, these promising results show that the presented technique is a feasible option for surgical treatment of acute proximal hamstring ruptures in older patients. These results need to be confirmed in clinical trials involving more patients.
Strength and limitations
In this study, we not only reported on patient reported outcome in patients after hamstring repair but also included objective quantitative functional parameters. Combining subjective satisfaction results and clinical scores with muscle strength measurements provided important insight into the outcome of a modified surgical technique. Because hamstring rupture is not a very common injury, only 17 patients were treated between 2014 and 2019 of which 13 volunteered to participate in this retrospective case series. Our patients were older than the populations in previous studies, were not athletically ambitious, and had a large variation in body mass index. Hence, the results of our study may not be readily transferrable to younger populations or athletes. As all patients at our clinic were treated with the modified procedure, we were not able to include a control group and hence we compared our results with those reported in the literature. The fact that all patients were treated by the same surgeon can be seen as strength and limitation as the treatment in this study was consistent but the influence of surgeon remains unknown. Outcome parameters were not assessed preoperatively because of the acute nature of the injuries. Nonetheless, the data presented here are promising and provide initial evidence that the described modified surgical technique produces good clinical and functional outcome in older patients.
Conclusion
After surgical repair of acute hamstring rupture using an off-anatomical insertion cite, older patients were very satisfied and had good clinical and functional outcome. Compared to the contralateral side, patients had comparable passive range of motion, muscle strength and thigh girth on their operated side. These results suggest that the modified surgical technique is feasible and results in excellent outcome that may be similar or even superior to those of established surgical techniques in older patients after acute hamstring rupture.
Data availability
The data that support the findings of this study are available from the corresponding author (KS)], upon reasonable request.
References
Cloke D, Moore O, Shah T, Rushton S, Shirley MD, Deehan DJ (2012) Thigh muscle injuries in youth soccer: Predictors of recovery. Am J Sports Med 40:433–439. https://doi.org/10.1177/0363546511428800
Ribeiro-Alvares J, Dornelles M, Fritsch C, Lima-e-Silva F, Medeiros T, Severo L, Marques V, Baroni B (2019) Prevalence of hamstring strain injury risk factors in professional and under-20 male football (soccer) players. J Sport Rehab 29:1–23. https://doi.org/10.1123/jsr.2018-0084
Silvers-Granelli HJ, Cohen M, Espregueira-Mendes J, Mandelbaum B (2021) Hamstring muscle injury in the athlete: State of the art. J ISAKOS 6:170–181. https://doi.org/10.1136/jisakos-2017-000145
Chen X, Sanchez GN, Schnitzer MJ, Delp SL (2016) Changes in sarcomere lengths of the human vastus lateralis muscle with knee flexion measured using in vivo microendoscopy. J Biomech 49:2989–2994. https://doi.org/10.1016/j.jbiomech.2016.07.013
Diemer WM, Winters M, Tol JL, Pas H, Moen MH (2021) Incidence of acute hamstring injuries in soccer: A systematic review of 13 studies involving more than 3800 athletes with 2 million sport exposure hours. J Orthop Sports Phys Ther 51:27–36. https://doi.org/10.2519/jospt.2021.9305
Cohen S, Bradley J (2007) Acute proximal hamstring rupture. J Am Acad Orthop Surg 15:350–355. https://doi.org/10.5435/00124635-200706000-00004
Petchprapa CN, Bencardino JT (2013) Tendon injuries of the hip. Magn Reson Imaging Clin N Am 21:75–96. https://doi.org/10.1016/j.mric.2012.09.004
Degen RM (2019) Proximal hamstring injuries: Management of tendinopathy and avulsion injuries. Curr Rev Musculoskelet Med 12:138–146. https://doi.org/10.1007/s12178-019-09541-x
Brucker PU, Imhoff AB (2005) Functional assessment after acute and chronic complete ruptures of the proximal hamstring tendons. Knee Surg Sports Traumatol Arthrosc 13:411–418. https://doi.org/10.1007/s00167-004-0563-z
Gidwani S, Bircher MD (2007) Avulsion injuries of the hamstring origin - a series of 12 patients and management algorithm. Ann R Coll Surg Engl 89:394–399. https://doi.org/10.1308/003588407X183427
Lempainen L, Sarimo J, Heikkila J, Mattila K, Orava S (2006) Surgical treatment of partial tears of the proximal origin of the hamstring muscles. Br J Sports Med 40:688–691. https://doi.org/10.1136/bjsm.2006.028191
Pasic N, Giffin JR, Degen RM (2020) Practice patterns for the treatment of acute proximal hamstring ruptures. Phys Sportsmed 48:116–122. https://doi.org/10.1080/00913847.2019.1645576
Bodendorfer BM, Curley AJ, Kotler JA, Ryan JM, Jejurikar NS, Kumar A, Postma WF (2018) Outcomes after operative and nonoperative treatment of proximal hamstring avulsions: A systematic review and meta-analysis. Am J Sports Med 46:2798–2808. https://doi.org/10.1177/0363546517732526
Best R, Eberle J, Beck F, Huth J, Becker U (2017) surgical refixation after proximal hamstring tendon avulsion injuries: Does the time of surgery influence functional outcomes? Sportverletz Sportschaden 31:160–166. https://doi.org/10.1055/s-0043-113211
Birmingham P, Müller M, Wickiewicz T, Cavanaugh J, Rodeo S, Warren R (2011) Functional outcome after repair of proximal hamstring avulsions. J Bone Joint Surg Am 93:1819–1826. https://doi.org/10.2106/JBJS.J.01372
Arner JW, McClincy MP, Bradley JP (2019) Hamstring injuries in athletes: Evidence-based treatment. J Am Acad Orthop Surg 27:868–877. https://doi.org/10.5435/JAAOS-D-18-00741
Harris JD, Griesser MJ, Best TM, Ellis TJ (2011) Treatment of proximal hamstring ruptures - a systematic review. Int J Sports Med 32:490–495. https://doi.org/10.1055/s-0031-1273753
Obey MR, Broski SM, Spinner RJ, Collins MS, Krych AJ (2016) Anatomy of the adductor magnus origin: Implications for proximal hamstring injuries. Orthop J Sports Med 4:2325967115625055. https://doi.org/10.1177/2325967115625055
Arner JW, Freiman H, Mauro CS, Bradley JP (2019) Functional results and outcomes after repair of partial proximal hamstring avulsions at midterm follow-up. Am J Sports Med 47:3436–3443. https://doi.org/10.1177/0363546519879117
Aldridge SE, Heilpern GN, Carmichael JR, Sprowson AP, Wood DG (2012) Incomplete avulsion of the proximal insertion of the hamstring: Outcome two years following surgical repair. J Bone Joint Surg Br 94:660–662. https://doi.org/10.1302/0301-620X.94B5.28043
Pietrzak JR, Kayani B, Tahmassebi J, Haddad FS (2018) Proximal hamstring tendinopathy: Pathophysiology, diagnosis and treatment. Br J Hosp Med (London) 79:389–394. https://doi.org/10.12968/hmed.2018.79.7.389.
Costa LP, Barros AAG, Vassalo CC, Sonnery-Cottet B, Barbosa VAK, Temponi EF (2016) A new technique for surgical treatment of proximal hamstring tendinopathy in a triathlon athlete. J Orthop Case Rep 6:69–72. https://doi.org/10.13107/jocr.2250-0685.638.
Kotler DH, Babu AN, Robidoux G (2016) Prevention, evaluation, and rehabilitation of cycling-related injury. Curr Sports Med Rep 15:199–206. https://doi.org/10.1249/JSR.0000000000000262
Shrestha N, Kukkonen-Harjula KT, Verbeek JH, Ijaz S, Hermans V, Pedisic Z (2018) Workplace interventions for reducing sitting at work. Cochrane Database System Rev. https://doi.org/10.1002/14651858.CD010912.pub5
van der Made AD, Reurink G, Gouttebarge V, Tol JL, Kerkhoffs GM (2015) Outcome after surgical repair of proximal hamstring avulsions: A systematic review. Am J Sports Med 43:2841–2851. https://doi.org/10.1177/0363546514555327
Carmichael J, Packham I, Trikha SP, Wood DG (2009) Avulsion of the proximal hamstring origin. J Bone Joint Surg Am 91:249–256
Rowen DA, Likens AD, Stergiou N (2020) Revisiting a classic: Muscles, reflexes, and locomotion by mcmahon. Biomech Gait Analysis. https://doi.org/10.1016/b978-0-12-813372-9.00006-3
MacIntosh BR (2017) Recent developments in understanding the length dependence of contractile response of skeletal muscle. Eur J Appl Physiol 117:1059–1071. https://doi.org/10.1007/s00421-017-3591-3
Rassier DE (2017) Sarcomere mechanics in striated muscles: From molecules to sarcomeres to cells. Am J Physiol Cell Physiol 313:C134–C145. https://doi.org/10.1152/ajpcell.00050.2017
Minozzo FC, Lira CABd (2013) Muscle residual force enhancement: A brief review. Clinics (Sao Paulo, Brazil) 68:269–274. https://doi.org/10.6061/clinics/2013(02)r01
Blakeney WG, Zilko SR, Edmonston SJ, Schupp NE, Annear PT (2017) Proximal hamstring tendon avulsion surgery: Evaluation of the perth hamstring assessment tool. Knee Surg Sports Traumatol Arthrosc 25:1936–1942. https://doi.org/10.1007/s00167-016-4214-y
Rabin R, de Charro F (2001) Eq-sd: A measure of health status from the euroqol group. Ann Med 33:337–343
Dingemans SA, Kleipool SC, Mulders MAM, Winkelhagen J, Schep NWL, Goslings JC, Schepers T (2017) Normative data for the lower extremity functional scale (lefs). Acta Orthop 88:422–426. https://doi.org/10.1080/17453674.2017.1309886
Wichman D, Rasio JP, Looney A, Nho SJ (2021) Physical examination of the hip. Sports Health 13:149–153. https://doi.org/10.1177/1941738120953418
Chevillotte CJ, Ali MH, Trousdale RT, Pagnano MW (2009) Variability in hip range of motion on clinical examination. J Arthroplasty 24:693–697. https://doi.org/10.1016/j.arth.2008.04.027
Polkowski GG, MaJCC MD (2010) Hip biomechanics. Sports Med Arthrosc Rev 18:56–62
Drouin JM, Valovich-mcLeod TC, Shultz SJ, Gansneder BM, Perrin DH (2004) Reliability and validity of the biodex system 3 pro isokinetic dynamometer velocity, torque and position measurements. Eur J Appl Physiol 91:22–29. https://doi.org/10.1007/s00421-003-0933-0
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, Consortium RE (2019) The redcap consortium: Building an international community of software platform partners. J Biomed Inform 95:103208. https://doi.org/10.1016/j.jbi.2019.103208.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (redcap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. https://doi.org/10.1016/j.jbi.2008.08.010
McGrath TM, Waddington G, Scarvell JM, Ball NB, Creer R, Woods K, Smith D (2016) The effect of limb dominance on lower limb functional performance–a systematic review. J Sports Sci 34:289–302. https://doi.org/10.1080/02640414.2015.1050601
Best R, Eberle J, Beck F, Beckmann J, Becker U (2019) Functional impairment after successful surgical reconstruction for proximal hamstring avulsion. Int Orthop 43:2341–2347. https://doi.org/10.1007/s00264-018-4263-6
Best R, Meister A, Meier M, Huth J, Becker U (2021) Predictive factors influencing functional results after proximal hamstring tendon avulsion surgery: A patient-reported outcome study after 227 operations from a single center. Orthop J Sports Med 9:23259671211043096. https://doi.org/10.1177/23259671211043097
Pihl E, Jonsson KB, Berglöf M, Brodin N, Sköldenberg O, Hedbeck CJ (2021) Exploring the perth hamstring assessment tool and lower extremity functional scale in a proximal hamstring avulsion cohort: A cross-sectional study. Am J Sports Med 49:1732–1740. https://doi.org/10.1177/03635465211008568
Cohen SB, Rangavajjula A, Vyas D, Bradley JP (2012) Functional results and outcomes after repair of proximal hamstring avulsions. Am J Sports Med 40:2092–2098. https://doi.org/10.1177/0363546512456012
Wegrzynek PA, Wainwright E, Ravalier J (2020) Return to work interventions for chronic pain: A systematic review. Occup Med (Lond) 70:268–277. https://doi.org/10.1093/occmed/kqaa066
Cullen KL, Irvin E, Collie A, Clay F, Gensby U, Jennings PA, Hogg-Johnson S, Kristman V, Laberge M, McKenzie D, Newnam S, Palagyi A, Ruseckaite R, Sheppard DM, Shourie S, Steenstra I, Van Eerd D, Amick BC 3rd (2018) Effectiveness of workplace interventions in return-to-work for musculoskeletal, pain-related and mental health conditions: An update of the evidence and messages for practitioners. J Occup Rehabil 28:1–15. https://doi.org/10.1007/s10926-016-9690-x
Grant M, J OB-E, Froud R, Underwood M, Seers K (2019) The work of return to work Challenges of returning to work when you have chronic pain: A meta-ethnography. BMJ Open 9:e025743. https://doi.org/10.1136/bmjopen-2018-025743
Wood D, French SR, Munir S, Kaila R (2020) The surgical repair of proximal hamstring avulsions. Bone Joint J 102-b:1419–1427. https://doi.org/10.1302/0301-620x.102b10.Bjj-2019-1112.R1.
Fouasson-Chailloux A, Menu P, Mesland O, Dauty M (2020) Strength assessment after proximal hamstring rupture: A critical review and analysis. Clin Biomech (Bristol, Avon) 72:44–51. https://doi.org/10.1016/j.clinbiomech.2019.11.016
Reza T, Hinkle AJ, Perez-Chaumont A, Brown SM, Mulcahey MK (2021) Systematic review of outcome measures used after proximal hamstring repair. Orthop J Sports Med 9:23259671211005100. https://doi.org/10.1177/23259671211005101
Funding
Open access funding provided by University of Basel. This study was funded by Department of Orthopedics and Traumatology of the University Hospital Basel, Switzerland. Linda Bühl’s salary was partially funded by a research fellowship by Zimmer Biomet Deutschland GmbH. The sponsor did not have a role in any aspect of this study.
Author information
Authors and Affiliations
Contributions
TC performed the clinical exams, entered data and drafted the manuscript; LB collected, processed, analyzed and interpreted the data and critically revised the manuscript; CN supervised data collection and monitored data quality control and critically revised the manuscript; NB collected, processed and entered data; AM analyzed and interpreted the data and drafted the manuscript; KS defined the research question, performed the surgery, and critically revised the manuscript; all authors approved the final draft of the manuscript and are accountable for all aspects of the work. All authors had full access to all data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by Ethikkommission Nordwest- und Zentralschweiz (EKNZ 2021–02276) on 11. November 2020.
Informed consent
All participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chocholáč, T., Bühl, L., Nüesch, C. et al. Modified surgical anchor refixation in older patients with acute proximal hamstring rupture: clinical outcome, patient satisfaction and muscle strength. Arch Orthop Trauma Surg 143, 4679–4688 (2023). https://doi.org/10.1007/s00402-022-04752-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-022-04752-3