Abstract
Purpose
Muscular strength loss and atrophy are postoperative complications. This systematic review with meta-analysis investigated the course of on knee extensor mass and strength from pre-surgery over total knee arthroplasty to rehabilitation and recovery.
Methods
A systematic literature search was conducted in PubMed (Medline), Cochrane Library (CINAHL, Embase) and Web of Science (until 29th of June 2022). Main inclusion criteria were ≥ 1 preoperative and ≥ 1 measurement ≥ 3-months post-operation and ≥ 1 objective assessment of quadriceps strength, muscle mass or neuromuscular activity, measured at both legs. Studies were excluded if they met the following criteria: further impairment of treated extremity or of the contralateral extremity; further muscle affecting disease, or muscle- or rehabilitation-specific intervention. The Robins-I tool for non-randomized studies, and the Cochrane Rob 2 tool for randomized controlled studies were used for risk of bias rating. Pre-surgery, 3 months, 6 months and 1 year after surgery data were pooled using random effects meta-analyses (standardized mean differences, SMD, Hedge’s g) in contrast to the pre-injury values.
Results
1417 studies were screened, 21 studies on 647 participants were included. Thereof, 13 were non-randomized controlled trails (moderate overall risk of bias in most studies) and 7 were randomized controlled trials (high risk of bias in at least one domain in most studies). Three (k = 12 studies; SMD = − 0.21 [95% confidence interval = − 0.36 to − 0.05], I2 = 4.75%) and six (k = 9; SMD = − 0.10 [− 0.28 to − 0.08]; I2 = 0%) months after total knee arthroplasty, a deterioration in the strength of the operated leg compared with the strength of the non-operated leg was observed. One year after surgery, the operated leg was stronger in all studies compared to the preoperative values. However, this increase in strength was not significant compared to the non-operated leg (k = 6, SMD = 0.18 [− 0.18 to 0.54], I2 = 77.56%).
Conclusion
We found moderate certainty evidence that deficits in muscle strength of the knee extensors persist and progress until 3 months post-total knee arthroplasty in patients with end-stage knee osteoarthritis. Very low certainty evidence exists that preoperatively existing imbalance of muscle strength and mass in favor of the leg not undergoing surgery is not recovered within 1 year after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) of the knee is associated with decreased functional activity, progressive knee pain and severe immobilization-induced skeletal muscle atrophy [10]. The surgical restoration of joint function by total knee arthroplasty (TKA) is the last option to decrease subjective pain and enhance quality of life in concerned patients.
The rehabilitation after TKA is often accompanied by long-term deficits in skeletal muscle health (SMH) such as muscle atrophy, strength losses and impaired neuromuscular activity [13]. These deficits are most often already present preoperatively [10] and exacerbate during the surgical procedure and subsequent hospitalization [32]. Although state-of-the art rehabilitation concepts try to reduce postoperative declines in SMH and support joint function, muscular deficits continue to develop progressively and can be demonstrated even years after TKA [24, 47]. Since it is known that preoperative muscle strength is associated with good performance outcomes after TKA [34, 58], the importance of skeletal muscle health for the success of the post-operative therapy is given.
Knowing the course of muscle strength in a standard care population would be helpful to rate the effectiveness of interventional trials on pre- and postoperative rehabilitation measures in TKA and, from a practical point-of-view, to identify patients with a below-mean deficit; these could be treated more deficit-orientated.
Since the usual course of muscle mass and strength following surgical therapy and subsequent standard rehabilitation is unknown, this systematic review with meta-analysis investigated the course of on knee extensor mass and strength from pre-surgery over total knee arthroplasty to rehabilitation and recovery.
Methods
Inclusion criteria
Studies were eligible for inclusion if they met the following criteria: (a) measurement of the quadriceps femoris muscle strength or measurement of muscle mass or measurement of neuromuscular activity; (b) measurement in both legs; (c) standardized measurement technique (d) study text available in German or English, (e) preoperative values of at least one outcome of interest reported; (f) follow-up measurements at least 3 months post-surgery; (g) maximum effort quadriceps strength measurement; (h) randomized controlled trial or cohort study.
Exclusion criteria
Studies were excluded if they met following criteria: (a) further or secondary impairment(s) of the treated extremity; (b) additional impairment of the contralateral extremity; (c) further muscle affecting disease (neurological, rheumatological, oncological); (d) muscle- or rehabilitation- specific interventions beyond the medically prescribed formal standard care/rehabilitation (such as exercise interventions as part of a therapy RCT). (e) strength values taken from questionnaires; (f) strength values just measuring angle to extension maximal force momentum.
Information sources
The following databases were used for searching: The Cochrane library (CENTRAL, including EMBASE and CINAHL), Web of Science, and PubMed; from January 2000 to June 2022. From the studies included, all reference lists were screened for further eligible studies.
Search strategy
To find appropriate studies the following eight searching combinations, with the database-specific Boolean operators, were entered in each database: 1. “total knee arthroplasty” AND “muscle mass” 2. “total knee arthroplasty” AND “strength” 3. “total knee arthroplasty” AND “neuromuscular activation” 4. “total knee arthroplasty” AND “contralateral leg” 5. “total knee replacement” AND “muscle mass” 6. “total knee replacement” AND “strength” 7. “total knee replacement „AND “neuromuscular activation” 8. “total knee replacement” AND “contralateral leg”.
Selection process
Each step of the selection process was done by two independent examiners (RS, AF). The comparison of the studies was done at the full-text-retrieval step. Disparities in the included studies were discussed; a third reviewer (MB) was included if no consent could be found.
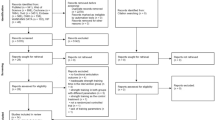
Selection of studies is shown in the PRISMA Flow Chart (Fig. 1). After deleting duplicate articles, the titles were assessed according to the previously determined inclusion and exclusion criteria. Afterwards the examiners analyzed the abstracts of the studies. The full texts for the abstract considered eligible were afterwards retrieved. If studies were not obtainable, the corresponding authors were contacted via email. Data were extracted from the full text of studies included in the systematic review. Duplicate removal and comparison between the two reviewers was done using Microsoft Excel (Microsoft Corporation, Version 16.47.1).
Data collection process
Data collection was done by the same two reviewers using the same procedure as during the selection process. Both researchers read all studies and extracted all possible data into one overview table, in Excel (Microsoft Corporation, Version 16.47.1), each. After data extraction both overview tables were compared and merged.
Data items
At the beginning of the data extraction following data were collected and summarized in an Excel table (Microsoft Corporation, Version 16.47.1):
-
General information (number of participants, patient characteristics, study characteristics).
-
Outcomes (strength measurements, muscle mass measurements, muscle activation, wellbeing, range of motion and performance measurements).
The (outcomes, dependent variables) data that had been extracted in addition to strength, muscle mass and neuromuscular activity were analyzed for potential subgroup analyses and meta regressions. For inclusion in the meta-analyses parameters had to be obtained with a standardized measurement tool, adequate techniques and analysis of both legs had to be done and taken at least in three studies at the same time point.
Study risk of Bias assessment
The Robins-I tool [43] was used to assess controlled non-randomized before and after studies. The Risk of Bias (RoB) 2 tool [44] was applied for randomized trials. Risk of Bias was rated by two independent researchers. After assessing all studies (i.e., outcomes) differences were resolved by discussion. Only data that were relevant for the systematic review were extracted from the individual studies.
Results from both analyses, Robins I and RoB 2, were converted from an Excel table (Microsoft Corporation, Version 16.54) with the robvis visualization tool [28] and displayed as a traffic light and as summary plots (Figs. 2, 3, 4 and 5).
Effect measures
Effect sizes for each outcome were calculated as standardized mean differences (SMD, pre- to post-surgery values, always in comparison to the contralateral control leg) in the form of Hedge’s g. For all outcomes with preoperative and postoperative values, for operated and non-operated leg, effect sizes were calculated.
Synthesis method
Measurements were selected at the closest time points before injury and 3 months, 6 months, or 1 year after surgery. For each of these timepoints, a pairwise (in comparison to the contralateral value) meta-analysis in comparison to the pre-surgery strength was calculated.
If mean and standard deviation could not directly be retrieved from the original study, data were imputed following the recommendations made by the Cochrane collaboration.
Main analyses
Effect size and its variance were used to perform forest plots and heterogeneity analyses in Jamovi (Version 1.8.2.0, jamovi.org, Sydney, Australia) using the module “Major”. The meta-analyses were performed with random effects models. Weighted standardized mean differences in the form of Hedge´s g were used as effect size estimators. Mean effect sizes and meta-analyses estimates (95% confidence interval, p value) were calculated for the analyses. Z-Statistics at a 5% alpha-error-probability were calculated to test for overall effects.
For the calculation of the between effects heterogeneity, Tau2, the maximum restricted likelihood approach was used. Besides, I2 was calculated for the between effects heterogeneity.
Multilevel meta-regression
A multilevel meta-regression was done in “R” (R Core Team, Version 1.4.1106, Vienna, Austria) to investigate whether the effect sizes correlate with the following independent moderators: Age, Body Mass Index (BMI), time after the operation (months) and the share of female participants. Meta-regression estimates, its standard error and the 95% confidence interval were calculated. Since the independent variables do not exhibit within-study variability, tau2 could not be calculated. Therefore, (Cochranes) Q was calculated to determine heterogeneity of the results. R2 was calculated to calculate how much of the heterogeneity in the meta-analyses can be explained by the moderators.
Sensitivity analysis
A sensitivity analysis was not conducted because all three meta-analyses consisted of an insufficient number of studies.
Reporting Bias assessment
Funnel plots were plotted and Egger’s regression tests to detect any funnel asymmetry were performed for reporting bias assessment. For both, Jamovi (The jamovi project (2021). jamovi (Version 1.6) [Computer Software]. Retrieved from https://www.jamovi.org) with the Modul “Major” was used. Reporting bias assessment was just reported for the 3 months analysis because it was the only meta-analysis consisting of ten studies [42].
Certainty assessment
The quality of evidence in each meta-analysis was evaluated with the “Grades of Research, Assessment, Development and Evaluation” (GRADE) approach. The evidence derived by the meta-analyses was classified in “high”, “moderate”, “low” or “very low”. Each certainty of evidence could then be up-or downgraded based on the following five criteria: 1. Risk of Bias; 2. Inconsistency; 3. Indirectness; 4. Imprecision; 5. Publication Bias.
Results
Study selection
The study flow is depicted in Fig. 1. Of the 24 studies assessed for eligibility, one was excluded because patients underwent pre-operative rehabilitation [53] and one study specified a strength measurement but just angle-to-extension maximal force momentum was reported [2]. One study [23] measured muscle mass only postoperatively. Finally, 21 studies were included (Fig. 1). 20 studies reported bilateral strength measurements and were included in the meta-analyses. One study measured bilateral leg lean tissue mass and was included in the systematic review but not in the meta-analyses [19].
Study characteristics
Detailed study characteristics are shown in Table 1. Moutzouri et al. [36] measured the strength values after fourteen weeks. These results were included in the meta-analysis 3 months after the surgery. Merk et al. [29] measured strength 5–7 months post-operatively. Those results were included in the 6 months postoperative analysis. Unlike other studies, Merk et al. [29] differentiated between contralateral leg with and without previous total knee replacement. In this review strength values from the contralateral leg without previous knee arthroplasty was used for meta-analyses.
Risk of Bias of individual studies
Non-randomized studies
An overview of all non-randomized studies is presented in Figs. 2 and 3. All matched before and after studies except for one [55] displayed moderate to serious risks for a bias due to confounding factors and due to not containing information about what patients did before their operation. Merk et al. [29] study was the only study with serious bias due to selection of participants because no exclusion criteria were reported. The possibility that patients with other diseases took part in that study cannot be ruled out. Besides an anamnesis survey and a thigh circumference measurement were made but results were not reported. That is why bias due to missing data was classified with serious risk of bias and bias in selection of the reported result was classified moderate.
The surgery technique was not mentioned in four studies which led to a moderate risk of bias due to deviations from the intended intervention [18, 19, 29, 56]. Four studies did not specify the order of measuring of the examined parameters. These studies were judged with moderate risk of bias in measurement of the outcomes [3, 18, 29, 34].
Randomized controlled studies
The risk of bias for the randomized controlled trials are demonstrated in Figs. 4 and 5. Four studies [22, 39, 49, 50] were judged with high risk of bias arising from the randomization process (no allocation concealment). Five studies [22, 36, 39, 49, 50] contained no intention to treat analysis which led to a judgment of some concerns in domain two. Tsukada et al. [50] lost seven patients in follow-up measurements. Two of them were lost because of vein thrombosis. If thrombosis resulted from the operation, an exclusion of these two patients would distort reported results. Hence investigators assessed this study with some concerns for bias due to missing outcome data. Six studies were classified with some concerns in risk of bias in measurement of the outcome because assessment have been influenced by the knowledge of the outcome [20, 22, 30, 39, 49, 50].
Result of the main syntheses: strength/torque
Meta-analysis for strength, neuromuscular activation and muscle mass were intended. Due to insufficient data, neuromuscular activation had to be excluded from this systematic review and muscle mass was only included in the systematic review but not in the meta-analyses.
Three months post-surgery
At 3 months, the pooled effect size was negative (Fig. 6).
The overall quadriceps force-progression in the operated leg was significantly less when comparing it to the non-operated leg (Fig. 6). Since all studies reported stronger non-operated legs than operated legs preoperatively, findings from the meta-analyses indicate an increase in the contralateral differences. This result is significant (Fig. 6). The presented values for the strength progression from preoperation until 3 months after the operation have little heterogeneity (Fig. 6).
Six months post-surgery
At 6 months after the operation, no significant pooled effects could be reported (Fig. 7). Eight out of nine studies included in meta-analysis reported no significant outcomes. The presented values for the strength evolution from preoperation until 6 months after the operation have no heterogeneity (Fig. 7).
One year post-surgery
One year after the surgery, the overall effect size was not significant (Fig. 8). The presented values for the force progression from pre-surgery to 1 year after surgery show a high heterogeneity (Fig. 8).
Results of the lean leg tissue mass progression
In all studies, except for one [39], the operated leg was found to be weaker than the contralateral leg before surgery. 6 months and 1 year after the surgery, the operated leg was stronger than before the operation in most studies (Table 1). However, after surgery, the lean mass in both legs decreased [19]. The initial decrease in muscle mass from pre-surgery to 6 months after surgery was slowed, but lean mass continued to decrease from 6 months to 1 year after surgery in both legs [19].
Meta-regression
All effect sizes except for one were included in the regression analysis. Moutzouris et al. [36] effect size was not included because the authors did not report information on the percentage of female participants and body mass index.
Results of the meta-regression are presented in Table 2. The variation in effect sizes can be attributed to a large extent to the examined independent variables (R2 = 0.31). No regressor significantly contributed to the effect size heterogeneity reduction.
Reporting Bias
Funnel plot (Fig. 9) and Egger’s Regression test (value = − 1312; p = 0.19) indicated no publication bias.
Certainty of evidence
Since all studies were evaluated with Robins I and Rob 2 the initial classification was “high certainty” for all three meta-analyses. Due to a serious risk in the domains “Bias due to confounding” in studies evaluated with the Robins I and “bias arising from randomization process” in the Rob 2 tool, due to the high heterogeneity (downgrade, only 1 year after the surgery), due to the large confidence interval of the effect size at 1 year and due to the lack of publication bias assessment (downgrade, 6 months and 1-year post-surgery), the certainty of evidence was finally as follows: Meta-analysis for 3 and 6 months were classified to contain moderate quality of evidence and the meta-analysis for 1 year after the operation was classified to contain very low quality of evidence.
Discussion
This systematic review with meta-analyses described and analyzed the course of skeletal muscle strength and mass of knee extensor muscles from preoperative to postoperative status until 1 year after a TKA intervention. We found very low certainty evidence that preoperatively existing imbalance of muscle strength and mass in favor of the leg not undergoing surgery is not counterbalanced within 1 year after primary TKA. Furthermore, the current results show, with moderate certainty evidence, that deficits in muscle strength of the knee extensors persist and progress until 3 months post-TKA persist in the operated leg until improvements can be detected.
In contrast to previously published meta-analyses, the present analyses only included studies that measured the operated leg and the contralateral leg as an intraindividual comparison. This approach was chosen because perceived pain scores and mobility in between-subject analyses are strongly affected by interindividual differences in sex, age, anthropometric characteristics, and activity level [25]. Therefore, present data are of significant practical importance for patients who are planning to undergo TKA and would like to know how their SMH is recovering during treatment.
As the preoperative muscle strength of the knee extensors, as well as the muscle mass and functional abilities of a patient, can be considered as positive predictive values for a successful rehabilitation, preoperative SMH has an important influence on the clinical outcome after TKA [8, 31]. As patient satisfaction after primary TKA is still only around 80% [5, 6], the results of our meta-analyses on the SMH of patients before and after TKA are most relevant for clinical care.
Preoperative muscle mass and strength
All 20 included studies reported differences between the to-be-operated leg and the contralateral leg in favor of the contralateral one. The largest contralateral strength difference was reported by Merk et al. [29], where the operated-leg had only half of the strength of the contralateral one. Prior to the surgery, the average operated-leg had only one-third of the strength of the non-operated leg.
Degenerative joint diseases are associated with a decrease in the skeletal muscles mass of the affected limb [1, 41].
In addition to immobility-related atrophy, decreased neuronal activation also appears to contribute to the loss of muscle mass. For example, some data indicate that degenerative knee joint changes lead to decreased excitability of motoneurons and thus to decreased activation of the quadriceps, which is referred to as arthrogenic muscle inhibition [21]. Therefore, pain associated reductions in mobility and neuromuscular activations are key elements in preoperative existing muscle mass and strength reductions.
Since surgical therapy, implants and postoperative rehabilitation strategies have already been modified considerably, some studies have focused on increasing the physical capacities of patients preoperatively to increase clinical outcome. Following the maxim “Better In, Better Out”, a variety of so-called “prehabilitation protocols” were investigated to modify SMH already before surgery [45]. However, recent meta-analyses on prehabilitation show only a low to moderate impact on pre- and postoperative outcomes [37, 57]. Generally, the problem is that a degenerative joint is not able to perform the mechanical stimulus that is necessary for muscle and strength gain without causing increased pain. Therefore, new training methods are being investigated that are able to build up muscle without a high mechanical component and thus improve the preoperative and postoperative outcome of TKA [12].
Postoperative rehabilitation of muscle mass and strength
Analysis of strength recovery after TKA-Surgery was done for 3-, 6 and 12 months postoperatively to demonstrate short- as well as long-term formal medically prescribed standard rehabilitation effects.
The results 3 months after TKA surgery showed that the total quadriceps force was significantly lower on the operated leg compared with the unoperated leg (Fig. 6). Since all studies reported stronger non-operated legs than operated legs preoperatively, present findings indicate an increase in the contralateral differences. These findings are well in line with the literature, describing the largest loss of muscle mass and muscle strength in the early phase of recovery after TKA [9, 11]. Since skeletal muscle tissue needs sufficient time to recover after traumatic events, whether of metabolic (e.g., muscle damage with atrophy induction due to ischemia/reperfusion injury through tourniquet use [27] or mechanical etiology (e.g., iatrogenic injury” [7], further progression after hospitalization could be caused by “immobility-induced atrophy” [16] and, “arthrogenic muscle inhibition after surgery” [32]. However, all studies that measured muscle strength at 3 and 6 months postoperatively reported enhancements for the operated leg [29, 30, 51, 52] (Table 1) which indicates that the surgery- and post-surgery-induced impairments are temporary and can be recovered. These findings are well in line with previous reported analysis, showing that postoperative muscle strength decreased 3 months after TKA and recovered to preoperative levels at 6 months post-surgery [35]. It must be noted that a negative effect size in the meta-analyses does not necessarily mean that the operated leg became weaker after surgery. It can also mean that the operated leg got stronger, but the strength increase was larger in the non-operated leg.
However, six out of seven studies included in our meta-analysis reported no significant outcomes for strength progression in comparison to the non-operated leg. As the overall effect size of the meta-analysis is slightly negative the preoperative contralateral differences cannot be balanced, it seems more that this disparity is increasing (Fig. 7). The results of long-term rehabilitation show that the operated leg becomes increasingly stronger, but the existing imbalance to the non-operated leg also increases up to 1 year after surgery (Table 1).
Several studies compared postoperative rehabilitation of muscle mass and muscle strength after TKA with age-matched healthy control patients. The results show that quadriceps strength still reaches only 70–80% of the strength of healthy controls 1 year after TKA [4]. Furthermore, results by Schache et al. [40] revealed that muscular weakness is continuously evident up to 3 years after surgery in comparison to healthy controls. The authors concluded that improving muscle strength postoperatively to a level similar to that of healthy control participants could improve patient dissatisfaction after TKA. However, our data show that 1 year after TKA, most patients are not even able to reach a level of muscle strength that is close to their non-operated leg. A comprehensive comparison of the operated to the non-operated leg has so far been considered as a research desideratum due to the limited data available [40]. Consequently, with appreciation of our data, it becomes apparent that the objective of rehabilitation interventions should be to match the muscle strength of both legs in the first place.
In addition to aforementioned results, present analyses demonstrate that certain physical characteristics have a negative influence on regeneration after TKA (Table 2). Studies with a higher percentage of female patients report a poorer strength progression of the operated leg compared to the non-operated leg (Table 2). These findings stay in contrast to research articles, describing a faster improvement in WOMAC score for woman after primary TKA [26]. However, our results suggest that standard care (surgery + standard rehabilitation) does not have a significant impact on muscular strength of the operated leg of women. Therefore, a special training program which focuses on muscle strength, hypertrophy and neuromuscular activation for women could be a useful postoperative tool to increase muscle strength and satisfaction after primary TKA.
Additionally, a negative correlation between higher BMI and strength progression after the surgery was found (Table 2). A higher BMI led to a lower strength progression of the operated leg compared to the contralateral leg. In fact, overweight patients tend to build up less muscular strength in the operated leg after TKA [38]. However, a recent review by Godziuk et al. [15] concluded that there is currently no evidence to support the benefit of preoperative weight loss on postoperative outcomes after TKA. Due to the lack of representative research, preoperative weight loss is further an individual therapeutical step between surgeon and patient. Nevertheless, based on our data, it appears that an increased BMI has a negative effect on muscular regeneration. Therefore, special pre- or postoperative treatment protocols with the aim to reduce weight and strengthen the muscles of the lower extremities could be a promising tool to enhance SMH rehabilitation after TKA.
In the comparison 1 year after TKA, it is particularly interesting that the confidence intervals of the effect sizes of the meta-analyses do not overlap, in contrast to the other two time points (Fig. 8). This indicates that patients’ strength progression in the studies is very different and thus the confidence interval of the overall result is very large (pooled effect size = 0.15; CI 95% − 0.30, 0.61) (Fig. 8). However, due to the positive overall effect size, it can be verified that after 1-year post-TKA the differences between the legs become smaller and the strength of the operated leg improves (Fig. 8).
Methodical procedure
The present results are based on reported outcomes from 20 included studies. As examination of the contralateral leg is performed infrequently, little information on differences between the operated and non-operated leg is available. Additionally, only six studies could be included for comparison after 1 year, which implies that no publication bias assessment could be done because of a lack of studies. These results show a significant research desideratum and highlight the importance for more studies including the contralateral leg in TKA, as well as longer study periods to monitor muscular regeneration.
Furthermore, difficulties were experienced in collecting the data for calculating the meta-analyses. Whereas some studies reported only the standard error, confidence interval or the interquartile range instead of reporting mean and standard deviations. Other studies reported the SD in the form of bar charts, which indicate that necessary standard deviation was extracted by software tools, here Web Plot Digitizer (Table 1).
In the interpretation of the results, another problem resulted from the fact that many studies showed an extensive wide confidence interval (Table 1). The explanation of this relates to the very individual rehabilitation of the patients included. The pattern of rehabilitation seems to be not linear, while some rehabilitate quickly, others still face long-term problems. In future studies, daily physical activity of the patients has to be considered to investigate differences in rehabilitation after TKA.
Although it is known that preoperative SMH is important for rehabilitation after TKA only one study reported preoperative trends [16] (Fig. 2). Preoperative fitness level seems to have a significant impact on rehabilitation quality. We suggest that preoperative exercise therapy could have a unique impact on recovery after surgery and postoperative activity. However, this has also not been investigated and should be focused in future studies.
The irregularities presented in the results of this systematic review can be explained by the fact that after standard rehabilitation the amount of exercise was not standardized anymore. Patients who were physically more active obviously gained more strength. Additionally, the two influencing variables BMI and female gender have a major impact on muscular regeneration after surgery.
Conclusion and relevance for practice
In conclusion, present data suggest that strength progression after primary TKA is not consistent and linear. Although on average the operated-leg got stronger from 3 months after the operation onwards, no significant improvement of the operated-leg strength compared to the non-operated leg was found, until 1 year after TKA. Since one of the postoperative goals of this surgical intervention is to rebuild muscular function, muscular strength and especially the treatment of existing strength imbalances, standard rehabilitative care does not seem to be sufficient.
Future studies should try to focus on how preoperative imbalances affect postoperative outcomes and which interventions can be applied pre- as well as postoperatively to reduce existing imbalances.
Considering, that SMH is currently only a secondary therapy goal of a TKA intervention, beyond pain reduction and restoration of physiological mobility, its impact on postoperative outcome can be counted as significant. Therefore, pre- and postoperative improvements in SMH could be a successful tool to enhance clinical outcomes and patient satisfaction after TKA.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data underlying all Figures and Tables are provided as a Source.
Change history
21 February 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00402-023-04808-y
References
Aily JB, de Noronha M, de Almeida AC, Pedroso MG, Maciel JG, Mattiello-Sverzut AC, Mattiello SM (2019) Evaluation of vastus lateralis architecture and strength of knee extensors in middle-aged and older individuals with knee osteoarthritis. Clin Rheumatol 38(9):2603–2611
Anchuela J, Gomez-Pellico L, Ferrer-Blanco M, Slocker M, Rodriguez R (2001) Muscular function and bone mass after knee arthroplasty. Int Orthop 25(4):253–256
Bascuas I, Tejero M, Monleón S, Boza R, Muniesa JM, Belmonte R (2013) Balance 1 year after TKA: correlation with clinical variables. Orthopedics 36(1):e6-12
Berghmans DDP, Lenssen AF, Emans PJ, de Bie RA (2018) Functions, disabilities and perceived health in the first year after total knee arthroplasty; a prospective cohort study. BMC Musculoskelet Disord 19(1):250
Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KDJ (2010) Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop 468(1):57–63
Canovas F, Dagneaux L (2018) Quality of life after total knee arthroplasty. Orthop Traumatol Surg Res 104(1S):S41–S46
de Simone V, Demey G, Magnussen RA, Lustig S, Servien E, Neyret P (2012) iatrogenic popliteus tendon injury during total knee arthroplasty results in decreased knee function two to three years postoperatively. Int Orthop 36(10):2061–2065
Devasenapathy N, Maddison R, Malhotra R, Zodepy S, Sharma S, Belavy DL (2019) Preoperative quadriceps muscle strength and functional ability predict performance-based outcomes 6 months after total knee arthroplasty: a systematic review. Phys Ther 99(1):46–61
Dreyer HC (2016) Tourniquet use during knee replacement surgery may contribute to muscle atrophy in older adults. Exerc Sport Sci Rev 44(2):61–70
Dreyer HC, Strycker LA, Senesac HA, Hocker AD, Smolkowski K, Shah SN, Jewett BA (2013) Essential amino acid supplementation in patients following total knee arthroplasty. J Clin Invest 123(11):4654–4666
Dreyer HC, Owen EC, Strycker LA, Smolkowski K, Muyskens JB, Kirkpatrick TK, Christie AD, Kuehl KS, Lantz BA, Shah SN, Mohler CG, Jewett BA (2018) Essential amino acid supplementation mitigates muscle atrophy after total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. JB JS Open Access 3(2):e0006
Franz A, Queitsch FP, Behringer M, Mayer C, Krauspe R, Zilkens C (2018) Blood flow restriction training as a prehabilitation concept in total knee arthroplasty: a narrative review about current preoperative interventions and the potential impact of BFR. Med Hypotheses 110:53–59
Franz A, Becker J, Behringer M, Mayer C, Bittersohl B, Krauspe R, Zilkens C (2019) Skeletal muscle health in osteoarthritis andtotal joint replacement therapy: effects of prehabilitation on muscular rehabilitation. Dtsch Z Sportmed 6:145–152
Gapeyeva H, Buht N, Peterson K, Ereline J, Haviko T, Pääsuke M (2007) Quadriceps femoris muscle voluntary isometric force production and relaxation characteristics before and 6 months after unilateral total knee arthroplasty in women. Knee Surg Sports Traumatol Arthrosc 15(2):202–211
Godziuk K, Prado CM, Beaupre L, Jones CA, Werle JR, Forhan M (2021) A critical review of weight loss recommendations before total knee arthroplasty. Joint Bone Spine 88(2):105114
Häggmark T, Jansson E, Eriksson E (1981) Fiber type area and metabolic potential of the thigh muscle in man after knee surgery and immobilization. Int J Sports Med 2(1):12–17
Hashimoto S, Hatayama K, Terauchi M, Saito K, Higuchi H, Chikuda H (2020) Preoperative hand-grip strength can be a predictor of stair ascent and descent ability after total knee arthroplasty in female patients. J Orthop Sci 25(1):167–172
Ho KKW, Lau LCM, Chau WW, Poon Q, Chung KY, Wong RMY (2021) End-stage knee osteoarthritis with and without sarcopenia and the effect of knee arthroplasty—a prospective cohort study. BMC Geriatr. https://doi.org/10.1186/s12877-020-01929-6
Hopkins SJ, Toms AD, Brown M, Welsman JR, Ukoumunne OC, Knapp KM (2016) A study investigating short- and medium-term effects on function, bone mineral density and lean tissue mass post-total knee replacement in a Caucasian female post-menopausal population: implications for hip fracture risk. Osteoporos Int 27(8):2567–2576
Huber EO, Roos EM, Meichtry A, de Bie RA, Bischoff-Ferrari HA (2015) Effect of preoperative neuromuscular training (NEMEX-TJR) on functional outcome after total knee replacement: an assessor-blinded randomized controlled trial. BMC Musculoskelet Disord 16:101
Hurley MV, Scott DL, Rees J, Newham DJ (1997) Sensorimotor changes and functional performance in patients with knee osteoarthritis. Ann Rheum Dis 56(11):641–648
Husby VS, Foss OA, Husby OS, Winther SB (2018) Randomized controlled trial of maximal strength training vs. standard rehabilitation following total knee arthroplasty. Eur J Phys Rehabil Med 54(3):371–379
Kim HJ, Park HJ, Oh JB, Chang MJ, Kang S-B, Kim YK, Oh SH, Chang CB (2021) Retrospective study of relationship between vastus medialis volume on SPECT-CT and outcome of unilateral total knee arthroplasty. Medicine (Baltimore) 100(1):e24138
LaStayo PC, Meier W, Marcus RL, Mizner R, Dibble L, Peters C (2009) Reversing muscle and mobility deficits 1 to 4 years after TKA: a pilot study. Clin Orthop 467(6):1493–1500
Lauermann SP, Lienhard K, Item-Glatthorn JF, Casartelli NC, Maffiuletti NA (2014) Assessment of quadriceps muscle weakness in patients after total knee arthroplasty and total hip arthroplasty: methodological issues. J Electromyogr Kinesiol 24(2):285–291
Liebs TR, Herzberg W, Roth-Kroeger AM, Rüther W, Hassenpflug J (2011) Women recover faster than men after standard knee arthroplasty. Clin Orthop 469(10):2855–2865
Liu D, Graham D, Gillies K, Gillies RM (2014) Effects of tourniquet use on quadriceps function and pain in total knee arthroplasty. Knee Surg Relat Res 26(4):207–213
McGuinness LA, Higgins JPT (2021) Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12(1):55–61
Merk J, Winkler C, Best R, Horstmann T (2008) Joint function and strength in patients before and after knee replacement surgery. Dtsch Z Sportmed 59(6):136–140
Minns Lowe CJ, Barker KL, Holder R, Sackley CM (2012) Comparison of postdischarge physiotherapy versus usual care following primary total knee arthroplasty for osteoarthritis: an exploratory pilot randomized clinical trial. Clin Rehabil 26(7):629–641
Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L (2005) Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol 32(8):1533–1539
Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Early S-ML (2005) Quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am 87(5):1047–1053
Mizner RL, Petterson SC, Snyder-Mackler L (2005) Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther 35(7):424–436
Mizner RL, Petterson SC, Clements KE, Zeni JA, Irrgang JJ, Snyder-Mackler L (2011) Measuring functional improvement after total knee arthroplasty requires both performance-based and patient-report assessments: a longitudinal analysis of outcomes. J Arthroplasty 26(5):728–737
Moon Y-W, Kim H-J, Ahn H-S, Lee D-H (2016) Serial changes of quadriceps and hamstring muscle strength following total knee arthroplasty: a meta-analysis. PLoS ONE 11(2):e0148193
Moutzouri M, Coutts F, Gliatis J, Billis E, Tsepis E, Gleeson N (2019) Early initiation of home-based sensori-motor training improves muscle strength, activation and size in patients after knee replacement: a secondary analysis of a controlled clinical trial. BMC Musculoskelet Disord 20(1):231
Moyer R, Ikert K, Long K, Marsh J (2017) The value of preoperative exercise and education for patients undergoing total hip and knee arthroplasty: a systematic review and meta-analysis. JBJS Rev 5(12):e2
Rigamonti AE, de Col A, Tamini S, Cicolini S, Caroli D, de Micheli R, Tringali G, Abbruzzese L, Marazzi N, Cella SG, Sartorio A (2019) Multidisciplinary integrated metabolic rehabilitation in elderly obese patients: effects on cardiovascular risk factors, fatigue and muscle performance. Nutrients 11(6):1240
Şavkin R, Büker N, Güngör HR (2021) The effects of preoperative neuromuscular electrical stimulation on the postoperative quadriceps muscle strength and functional status in patients with fast-track total knee arthroplasty. Acta Orthop Belg 87(4):735–744
Schache MB, McClelland JA, Webster KE (2014) Lower limb strength following total knee arthroplasty: a systematic review. Knee 21(1):12–20
Shorter E, Sannicandro AJ, Poulet B, Goljanek-Whysall K (2019) Skeletal muscle wasting and its relationship with osteoarthritis: a mini-review of mechanisms and current interventions. Curr Rheumatol Rep 21(8):40
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JPT (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343:d4002
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A-W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Swank AM, Kachelman JB, Bibeau W, Quesada PM, Nyland J, Malkani A, Topp RV (2011) Prehabilitation before total knee arthroplasty increases strength and function in older adults with severe osteoarthritis. J Strength Cond Res 25(2):318–325
Takamura D, Iwata K, Sueyoshi T, Yasuda T, Moriyama H (2021) Relationship between early physical activity after total knee arthroplasty and postoperative physical function: are these related? Knee Surg Relat Res. https://doi.org/10.1186/s43019-021-00118-y
Thomas AC, Stevens-Lapsley JE (2012) Importance of attenuating quadriceps activation deficits after total knee arthroplasty. Exerc Sport Sci Rev 40(2):95–101
Thomas AC, Judd DL, Davidson BS, Eckhoff DG, Stevens-Lapsley JE (2014) Quadriceps/hamstrings co-activation increases early after total knee arthroplasty. Knee 21(6):1115–1119
Topp R, Swank AM, Quesada PM, Nyland J, Malkani A (2009) The effect of prehabilitation exercise on strength and functioning after total knee arthroplasty. PM R 1(8):729–735
Tsukada Y, Matsuse H, Shinozaki N, Takano Y, Nago T, Shiba N (2020) Combined application of electrically stimulated antagonist muscle contraction and volitional muscle contraction prevents muscle strength weakness and promotes physical function recovery after total knee arthroplasty: a randomized controlled trial. Kurume Med J 65(4):145–154
Vahtrik D, Gapeyeva H, Aibast H, Ereline J, Kums T, Haviko T, Märtson A, Schneider G, Pääsuke M (2012) Quadriceps femoris muscle function prior and after total knee arthroplasty in women with knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 20(10):2017–2025
Vahtrik D, Ereline J, Gapeyeva H, Pääsuke M (2014) Postural stability in relation to anthropometric and functional characteristics in women with knee osteoarthritis following total knee arthroplasty. Arch Orthop Trauma Surg 134(5):685–692
van Leeuwen DM, de Ruiter CJ, Nolte PA, de Haan A (2014) Preoperative strength training for elderly patients awaiting total knee arthroplasty. Rehabil Res Pract 2014:462750
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez M-G, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT-A, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, Vaccaro KC de, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, Leo D de, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo J-P, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer A-C, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KMV, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O'Donnell M, O'Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, Leòn FR de, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJC, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SRM, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh P-H, Zaidi AKM, Zheng Z-J, Zonies D, Lopez AD, Murray CJL, AlMazroa MA, Memish ZA. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England). 380(9859):2163–96
Vuorenmaa M, Ylinen J, Kiviranta I, Intke A, Kautiainen HJ, Mälkiä E, Häkkinen A (2008) Changes in pain and physical function during waiting time and 3 months after knee joint arthroplasty. J Rehabil Med 40(7):570–575
Wada O, Nagai K, Hiyama Y, Nitta S, Maruno H, Mizuno K (2016) Diabetes is a risk factor for restricted range of motion and poor clinical outcome after total knee arthroplasty. J arthroplasty 31(9):1933–1937
Wang L, Lee M, Zhang Z, Moodie J, Cheng D, Martin J (2016) Does preoperative rehabilitation for patients planning to undergo joint replacement surgery improve outcomes? A systematic review and meta-analysis of randomised controlled trials. BMJ Open 6(2):e009857
Yoshida Y, Mizner RL, Ramsey DK, Snyder-Mackler L (2008) Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin biomech (Bristol, Avon) 23(3):320–328
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no financial and personal relationships with third parties or organizations that could have inappropriately influenced the present work. The authors further state that no funding was received.
Ethical approval
Yes.
Informed consent
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singla, R., Niederer, D., Franz, A. et al. The course of knee extensor strength after total knee arthroplasty: a systematic review with meta-analysis and -regression. Arch Orthop Trauma Surg 143, 5303–5322 (2023). https://doi.org/10.1007/s00402-022-04750-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-022-04750-5