Abstract
Introduction
It remains unclear whether rheumatoid arthritis might be a cause of false positive of the histology for the diagnosis of prosthetic joint infection. Our aim was to evaluate the usefulness of the histology for the diagnosis of infection during hip and knee prosthesis revision in patients with rheumatoid arthritis.
Materials and methods
All patients with the diagnosis of rheumatoid arthritis (RA) undergoing hip or knee revision surgery (total or partial) were retrospectively reviewed. Positive histology was considered when ≥ 5 neutrophils per high-power field (400×) were found in at least five separate microscopic fields. Patients who presented ≥ 2 positive cultures for the same microorganism or the presence of fistula were considered as “true positives”.
Results
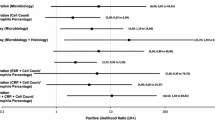
Thirty-two hip (n = 12) and knee (n = 20) revision procedures were performed. Sensitivity, specificity, positive and negative predictive value of the histology were 50%, 78.6%, 25% and 91.7%, respectively. Six out of the eight patients presenting with positive histology had negative cultures (75.0% of false positives).
Conclusions
Our results suggest that, in the context of RA, negative histological results have a very high negative predictive value. RA poses false positive histology results for the diagnosis of infection during hip and knee revision when conventional cultures are used for diagnosis of infection.
Similar content being viewed by others
References
Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J (2014) Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? ClinOrthopRelat Res 472(11):3254–3262
Parvizi J, Della Valle CJ (2010) AAOS clinical practice guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am AcadOrthopSurg 18(12):771–772
Bauer TW, Parvizi J, Kobayashi N, Krebs V (2006) Diagnosis of periprosthetic infection. J Bone JtSurg Am 88:869–882
Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N (2018) The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty 33(5):1309–1314
Kataoka M, Torisu T, Tsumura H, Yoshida S, Takashita M (2002) An assessment of histopathological criteria for infection in joint arthroplasty in rheumatoid synovium. ClinRheumatol 21(2):159–163
Muñoz-Mahamud E, Bori G, García S, Ramírez J, Riba J, Soriano A (2013) Usefulness of histology for predicting infection at the time of hip revision for the treatment of Vancouver B2periprosthetic fractures. J Arthroplasty 28(8):1247–1250
Bori G, Muñoz-Mahamud E, Garcia S, Mallofre C, Gallart X, Bosch J, Garcia E, Riba J, Mensa J, Soriano A (2011) Interface membrane is the best sample for histological study to diagnose prosthetic joint infection. Mod Pathol 24:579–584
Feldman DS, Lonner JH, Desai P, Zuckerman JD (1995) The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone JtSurg Am 77:1807–1813
Mirra JM, Amstutz HC, Matos M, Gold R (1976) The pathology of the joint tissues and its clinical relevance in prosthesis failure. ClinOrthopRelat Res 1976:221–240
Leone JM, Hanssen AD (2005) Management of infection at the site of a total knee arthroplasty. J Bone JtSurg Am 87:2335–2348
Morawietz L, Classen R, Schröder JH, Dynybil C, Perka C, Skwara A, Neidel J, Gehrke T, Frommelt L, Hansen T, Otto M, Barden B, Aigner T, Stiehl P, Schubert T, Meyer-Scholten C, König A, Ströbel P, Rader CP, Kirschner S, Lintner F, Rüther W, Bos I, Hendrich C, Kriegsmann J, Krenn V (2006) Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J ClinPathol 59:591–597
Pons M, Anglés F, Sánchez C, Matamala A, Cuchi E, Salavert M, Forcada P, Ferrer H (1999) Infected total hip arthroplasty–the value of intraoperative histology. Int Orthop 23:34–36
Athanasou NA, Pandey R, de Steiger R, Crook D, Smith PM (1995) Diagnosis of infection by frozen section during revision arthroplasty. J Bone JtSurg Br 77:28–33
Musso AD, Mohanty K, Spencer-Jones R (2003) Role of frozen section histology in diagnosis of infection during revision arthroplasty. Postgrad Med J 79:590–593
Ko PS, Ip D, Chow KP, Cheung F, Lee OB, Lam JJ (2005) The role of intraoperative frozen section in decision making in revision hip and knee arthroplasties in a local community hospital. J Arthroplasty 20:189–195
Kanner WA, Saleh KJ, Frierson HFJ (2008) Reassessment of the usefulness of frozen section analysis for hip and knee joint revisions. Am J ClinPathol 130:363–368
Della Valle CJ, Bogner E, Desai P, Lonner JH, Adler E, Zuckerman JD, Di Cesare PE (1999) Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone JtSurg Am 81:684–689
Bori G, Soriano A, García S, Mallofré C, Riba J, Mensa J (2007) Usefulness of histological analysis for predicting the presence of microorganisms at the time of reimplantation after hip resection arthroplasty for the treatment of infection. J Bone JtSurg Am 89:1232–1237
Bori G, Soriano A, García S, Gallart X, Casanova L, Mallofre C, Almela M, Martínez JA, Riba J, Mensa J (2006) Low sensitivity of histology to predict the presence of microorganisms in suspected aseptic loosening of a joint prosthesis. Mod Pathol 19:874–877
Abdul-Karim FW, McGinnis MG, Kraay M, Emancipator SN, Goldberg V (1998) Frozen section biopsy assessment for the presence of polymorphonuclear leukocytes in patients undergoing revision of arthroplasties. Mod Pathol 11:427–431
Tohtz SW, Müller M, Morawietz L, Winkler T, Perka C (2010) Validity of frozen sections for analysis of periprosthetic loosening membranes. ClinOrthopRelat Res 468:762–768
Spangehl MJ, Masri BA, O’Connell JX, Duncan CP (1999) Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone JtSurg Am 81:672–683
Pandey R, Drakoulakis E, Athanasou NA (1999) An assessment of the histological criteria used to diagnose infection in hip revision arthroplasty tissues. J ClinPathol 52:118–123
Banit DM, Kaufer H, Hartford JM (2002) Intraoperative frozen section analysis in revision total joint arthroplasty. ClinOrthopRelat Res 2002:230–238
Morawietz L, Tiddens O, Mueller M, Tohtz S, Gansukh T, Schroeder JH, Perka C, Krenn V (2009) Twenty-three neutrophil granulocytes in 10 high-power fields is the best histopathological threshold to differentiate between aseptic and septic endoprosthesis loosening. Histopathology 54:847–853
Bori G, Soriano A, García S, Gallart X, Mallofre C, Mensa J (2009) Neutrophils in frozen section and type of microorganism isolated at the time of resection arthroplasty for the treatment of infection. Arch Orthop Trauma Surg 129:591–595
Favetti F, Mazzotta G, Papalia M, Panegrossi G, Casella F, Falez F (2019) Contamination of revision procedures in patients with adverse tissues reaction to metal on metal implant. Eur Rev Med Pharmacol Sci 23(2):86–93
Cross A, Bakstad D, Allen JC, Thomas L, Moots RJ, Edwards SW (2005) Neutrophil gene expression in rheumatoid arthritis. Pathophysiology 12(3):191–202
Francés Borrego A, Martínez FM, Cebrian Parra JL, Grañeda DS, Crespo RG, López-Durán Stern L (2007) Diagnosis of infection in hip and knee revision surgery: intraoperative frozen section analysis. Int Orthop 31:33–37
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376(9746):1094–1108
Dayer JM (2003) The pivotal role of interleukin-1 in the clinical manifestations of rheumatoid arthritis. Rheumatology 42(Suppl 2):ii3–ii10
JavadMortazavi SM, Vegari D, Ho A, Zmitowski B, Parvizi J (2011) Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. ClinOrthopRelat Res 469:3049–3054
Kubista B, Hartzler RU, Wood CM, Osmon DR, Hanssen AD, Lewallen DG (2012) Reinfection after two-stage revision for periprosthetic infection of total knee arthroplasty. Int Orthop 36:65–71
Inagaki Y, Uchihara Y, Munemoto M, Scarborough M, Dodd CAF, Gibbons CLMH, Tanaka Y, Athanasou NA (2019) Correlation of histological and microbiological findings in septic and aseptic knee implant failure. Arch Orthop Trauma Surg 139(5):717–722
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of our center under the code HCB/2019/0042 and it does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Montoya-delaTorre, C., Fernández-Valencia, J.A., Martínez-Pastor, J.C. et al. Usefulness of histology for predicting infection at the time of hip and knee revision in patients with rheumatoid arthritis. Arch Orthop Trauma Surg 142, 2489–2495 (2022). https://doi.org/10.1007/s00402-021-03868-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-021-03868-2