Abstract
Introduction
Differences between tibial and femoral joint surfaces and knee compartments concerning coupled bone and cartilage turnover or bone–cartilage cross talk have not been previously examined, although the mechanical and biological interaction of the mineralized subchondral tissues with articular cartilage is of great importance for advancing osteoarthritis.
Materials and methods
Therefore, with the help of immunohistochemistry and real-time polymerase chain reaction (RT-PCR), human knee joint cartilage tissue was investigated for expression of key molecules of the extracellular matrix and cartilage composition (collagen type I and II, aggrecan) plus proteoglycan content (colorimetric analysis). Furthermore, we correlated the results with 3D microcomputed tomography of the underlying subchondral bone (high-resolution micro-CT system). Measurements were performed in dependence of the anatomical site (femoral vs. tibial, medial and lateral each) to identify regional differences during the osteoarthritic process. From an enduring series of 108 patients undergoing implantation of TKA, 34 osteochondral samples with lesions macroscopically classified as ICRS grade 1b (group A) and 34 samples with ICRS grade 3a/3b lesions (group B) were compared with 21 healthy controls.
Results
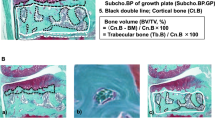
Concerning 3D analysis, the medial femoral condyle and tibia showed the most significant increase in bone volume fraction and a decrease in the trabecular number in group B frequently accompanied by subchondral bone resorption pits and enchondral ossification. Under physiological conditions, tibia plateaus show lower bone volume fraction than the corresponding femoral site and this difference enlarges with advancing OA. Partially even contradictory behavior was observed such as trabecular separation at the lateral tibial and medial plateau in osteochondral OA samples of the same patients. Collagen type II expression levels show faster and varying changes than type I during the OA process, leading to a lower positive or negative correlation with bone microstructural analysis, especially on the tibia plateau.
Conclusions
Structural bone and cartilage parameter changes showed varying developments and correlations among each other in the different compartments of the knee. As a clinical conclusion, therapies to postpone or prevent cartilage degeneration by influencing the loss of mineralized bone could be site dependent.





Similar content being viewed by others
References
Martínez-Calatrava MJ, Prieto-Potín I, Roman-Blas JA, Tardio L, Largo R, Herrero-Beaumont G (2012) RANKL synthesized by articular chondrocytes contributes to juxta-articular bone loss in chronic arthritis. Arthritis Res Ther 14:R149
Sims NA, Vrahnas C (2014) Regulation of cortical and trabecular bone mass by communication between osteoblasts, osteocytes and osteoclasts. Arch Biochem Biophys 561:22–28
Jahr H, Gunes S, Kuhn AR, Nebelung S, Pufe T (2019) Bioreactor-controlled physoxia regulates TGF-β signaling to alter extracellular matrix synthesis by human chondrocytes. Int J Mol Sci 20(7):E1715. https://doi.org/10.3390/ijms20071715
Sharma AR, Jagga S, Lee SS, Nam JS (2013) Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci 14:19805–19830
Dunn SL, Soul J, Anand S, Schwartz JM, Boot-Handford RP, Hardingham TE (2016) Gene expression changes in damaged osteoarthritic cartilage identify a signature of non-chondrogenic and mechanical responses. Osteoarthr Cartil 24:1431–1440
Everhart JS, Siston RA, Flanigan DC (2014) Tibiofemoral subchondral surface ratio (SSR) is a predictor of osteoarthritis symptoms and radiographic progression: data from the osteoarthritis initiative (OAI). Osteoarthr Cartil 22:771–778
Chokhandre S, Colbrunn R, Bennetts C, Erdemir A (2015) (2015) A comprehensive specimen-specific multiscale data set for anatomical and mechanical characterization of the tibiofemoral joint. PLoS ONE 10(9):e0138226. https://doi.org/10.1371/journal.pone.0138226.eCollection
Hoemann C, Kandel R, Roberts S, Saris DB, Creemers L, Mainil-Varlet P, Méthot S, Hollander AP, Buschmann MD (2011) International Cartilage Repair Society (ICRS) recommended guidelines for histological endpoints for cartilage repair studies in animal models and clinical trials. Cartilage 2:153–172
Aho OM, Finnilä M, Thevenot J, Saarakkala S, Lehenkari P (2017) Subchondral bone histology and grading in osteoarthritis. PLoS ONE 12(3):e01737–e1826
Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier J-P, Revell PA, Salter D, Path FRC, van den Ber WB (2006) Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil 14:13–29
Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FS, Olsvik PA, Penning LC, Toegel S (2010) MIQE precis: practical implementation of minimum standard guidelines for fluorescence -based quantitative realtime PCR experiments. BMC Mol Biol 11:74
Baelde HJ, Cleton-Jansen AM, Van Beerendonk H, Namba M, Bovee JVMG, Hogendoorn PCW (2001) High Quality RNA isolation from tumours with low cellularity and high extracellular matrix component for cDNA microarrays: application to chondrosarcoma. J Clin Pathol 54:778–782
Ruettger A, Neumann S, Wiederanders B, Huber R (2010) Comparison of different methods for preparation and characterization of total RNA from cartilage samples to uncover osteoarthritis in vivo. BMC Res Notes 3:7
Lahm A, Kasch R, Mrosek E, Spank H, Erggelet C, Esser J, Merk H (2012) Semiquantitative analysis of ECM molecules in the different cartilage layers in early and advanced osteoarthritis of the knee joint. Histol Histopathol 27:609–615
Anstaett OL, Brownlie J, Collins ME, Thomas CJ (2010) Validation of endogenous reference genes for RT-qPCR normalisation in bovine lymphoid cells (BL-3) infected with bovine viral diarrhoea virus (BVDV). Vet Immunol Immunopathol 137:201–207
Al-Sabah A, Stadnik P, Gilbert SJ, Duance VC, Blain EJ (2016) Importance of reference gene selection for articular cartilage mechanobiology studies. Osteoarthr Cartil 24:719–730
Squires GR, Okouneff S, Ionescu M, Poole RA (2003) The pathobiology of focal lesions development in aging human articular cartilage and molecular matrix changes characteristics of osteoarthritis. Arthr Rheum 48:1261–1270
Thomsen JS, Laib A, Koller B, Prohaska S, Mosekilde L, Gowin W (2005) Stereological measures of trabecular bone structure: comparison of 3D micro computed tomography with 2D histological sections in human proximal tibial bone biopsies. J Microsc 218:171–179
Hildebrand T, Laib A, Müller R, Dequeker J, Rüegsegger P (1999) Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res 14:1167–1174
Ding M, Odgaard A, Hvid I (2003) Changes in the three-dimensional microstructure of human tibial cancellous bone in early osteoarthritis. J Bone Joint Surg Br 85:906–912
Salmon PL, Ohlosson C, Shefelbine SJ, Doube M (2015) Structure model index does not measure rods and plates in trabecular bone. Frontiers in Endocrinolgy. https://doi.org/10.3389/fends.2015.00162
Teramoto T, Kamiya T, Sakurai T, Kanaya F (2018) Betti number ratios as quantitative indices for bone morphometry in three dimensions. Comput Methods Programs Biomed 162:93–98
Sanchez C, Deberg MA, Piccardi N, Msika P, Reginster JY, Henrotin YE (2005) Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by interleukin-6, -1β and oncostatin M pre-treated non-sclerotic osteoblasts. Osteoarthritis Cartilage 13:979–987
Lane NE, Thompson JM, Haupt D, Kimmel DB, Modin G, Kinney JH (1998) Acute changes in trabecular bone connectivity and osteoclast activity in the ovariectomized rat in vivo. J Bone Miner Res 13:229–236
Odgren PR, Witwicka H, Reyes-Gutierrez P (2016) The cast of clasts: catabolism and vascular invasion during bone growth, repair, and disease by osteoclasts, chondroclasts, and septoclasts. Connect Tissue Res 57:161–174
Cackowski FC, Anderson JL, Patrene KD, Choksi RJ, Shapiro SD, Windle JJ, Blair HC, Roodman GD (2010) Osteoclasts are important for bone angiogenesis. Blood 115:140–149
Vidal B, Pinto A, Galvão MJ, Santos AR, Rodrigues A, Cascão R, Abdulghani S, Caetano-Lopes J, Ferreira A, Fonseca JE, Canhao H (2012) Bone histomorphometry revisited. Acta Reumatol Port 37:294–300
Yagi R, McBurney D, Laverty D, Weiner S, Horton WE Jr (2005) Intrajoint comparisons of gene expression patterns in human osteoarthritis suggest a change in chondrocyte phenotype. J Orthop Res 23:1128–1138
Eckstein F, Hudelmaier M, Putz R (2006) The effects of exercise on human articular cartilage. J Anat 208:491–512
Le Graverand MP, Eggerer J, Vignon E, Otterness IG, Barclay L, Hart DA (2002) Assessment of specific mRNA levels in cartilage regions in a lapine model of osteoarthritis. J Orthop Res 20:535–544
Gouttenoire J, Bougault C, Aubert-Foucher E, Perrier E, Ronzière MC, Sandell L, Lundgren-Akerlund E, Mallein-Gerin F (2010) BMP-2 and TGF-beta1 differentially control expression of type II procollagen and alpha 10 and alpha 11 integrins in mouse chondrocytes. Eur J Cell Biol 89:307–314
Moulin D, Salone V, Koufany M, Clément T, Behm-Ansmant I, Branlant C, Charpentier B, Jouzeau JY (2017) MicroRNA-29b contributes to collagens imbalance in human osteoarthritic and dedifferentiated articular chondrocytes. Biomed Res Int. https://doi.org/10.1155/2017/9792512
Botter SM, Glasson SS, Hopkins B, Clockaerts S, Weinans H, van Leeuwen JP, van Osch GJ (2009) ADAMTS5−/− mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: implications for a link between cartilage and subchondral bone changes. Osteoarthr Cartil 17:636–645
Karsdal MA, Leeming DJ, Dam EB, Henriksen K, Alexandersen P, Pastoureau P, Altman RD, Christiansen C (2008) Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthr Cartil 16:638–646
Musumeci G, Szychlinska MA, Mobasheri A (2015) Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis: molecular markers of senescent chondrocytes. Histol Histopathol 30:1–12
Funding
There is no funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lahm, A., Dabravolski, D., Rödig, J. et al. Varying development of femoral and tibial subchondral bone tissue and their interaction with articular cartilage during progressing osteoarthritis. Arch Orthop Trauma Surg 140, 1919–1930 (2020). https://doi.org/10.1007/s00402-020-03480-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-020-03480-w