Abstract
Introduction
We assessed the efficacy of fibrin sealant (FS) and tranexamic acid (TXA) administered topically in patients with a hip fracture treated with prosthetic replacement.
Materials and methods
Parallel, multicentre, open label, randomised, clinical trial. We compared three interventions to reduce blood loss: (1) 10 ml of FS, (2) 1 g of topical TXA, both administered at the end of the surgery, and (3) usual haemostasis (control group). The main outcome was blood loss collected in drains. Other secondary variables were total blood loss, hidden blood loss, transfusion rate, average hospital stay, complications, adverse events, and mortality.
Results
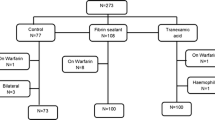
A total of 158 patients were included, 56 in the FS group, 52 in the TXA group, and 50 in the control group. The total amount of blood collected in drains was lower in the TXA group (148.6 ml, SD 122.7 in TXA; 168.2 ml, SD 137.4 in FS; and 201.5 ml, SD 166.5 in control group) without achieving statistical significance (p = 0.178). The transfusion rate was lower in the TXA group (32.7%), compared with FS group (42.9%) and control group (44.0%), without statistical significance (p = 0.341). There were no complications or adverse effects related to the evaluated interventions.
Conclusions
The use of TXA and FS administered topically prior to surgical closure in patients with a sub-capital femoral fracture undergoing arthroplasty did not significantly reduce either postoperative blood loss or transfusion rate, compared with a group that only received usual haemostasis.

Similar content being viewed by others
References
Bentler SE, Liu L, Obrizan M, Cook EA, Wright KB, Geweke JF, Chrischilles EA, Pavlik CE, Wallace RB, Ohsfeldt RL, Jones MP, Rosenthal GE, Wolinsky FD (2009) The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol 170:1290–1299
Singer BR, McLauchlan GJ, Robinson CM (1998) Epidemiology of fractures in 15000 adults: the influence of age and gender. J Bone Jt Surg Br 80:243–248
Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R et al (1996) Effect of anaemia and cardiovascular disease on surgical mortality and morbility. Lancet 348:1055–1060
Kirksey M, Lin Chiu Y, Ma Y, Gonzalez Della Valle A, Poultsides L, Gerner P, Memtsoudis SG (2012) Trends in in-hospital major morbidity and mortality after total joint arthroplasty: United States 1998–2008. Anesth Analg 115:321–327
Spahn DR (2010) Anemia and patient blood management in hip and knee surgery. A systematic review of the literature. Anesthesiology 113:482–495
Okamoto S, Hijikata-Okunomiya A, Wanaka K et al (1997) Enzyme controlling medicines: introduction. Semin Thromb Hemost 23:493–501
Brennan M (1991) Fibrin glue. Blood Rev 5:2404
Li J, Li HB, Zhai XC, Qin-Lei, Jiang XQ, Zhang ZH (2016) Topical use of topical fibrin sealant can reduce the need for transfusión, total blood loss and the volumen of drainage in total knee and hip arthroplasty: a systematic review and meta-analysis of 1489 patients. Int J Surg 36:127–137
Aguilera X, Martínez-Zapata MJ, Hinarejos P, Jordán M, Leal J, González JC, Monllau JC, Celaya F, Rodríguez-Arias A, Fernández JA, Pelfort X, Puig-Verdie Ll (2015) Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg 135:1017–1025
Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM (2014) A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Jt J 96:1005–1015
Wong J, Abrishami A, El Beheiry H, Mahomed N, Davey JR, Gandhi R et al (2010) Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty. J Bone Jt Surg Am 92:2503–2513
Molloy DO, Archbold HA, Ogonda L, McConway J, Wilson RK, Beverland DE (2007) Comparision of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomized controlled trial. J Bone Jt Surg Br 89:306–309
Mawatari M, Higo T, Tsutsumi Y, Shigematsu M, Hotokebuchi T (2006) Effectiveness of autologous fibrin tissue adhesive in reducing postoperative blood loss during total hip arthroplasty: a prospective randomised study of 100 cases. J Orthopaedic Surg 14:117–121
Sadeghi M, Mehr-Aein A (2007) Does a single bolus dose of tranexamic acid reduce blood loss and transfusion requirements during hip fracture surgery? A prospective study randomized double blind study in 67 patients. Acta Medica Iranica 45:437–442
Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, Mismetti P, Molliex S for the investigators of the tranexamic acid in hip-fracture surgery (THIF) study (2010) Tranexamic acid in hip fracture sugery: a randomized controlled trial. Br J Anaesth 104: 23–30
Nadler SB, Hidalgo JU. Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51:224–232
Mc Connell JS, Shewale S, Munro NA, Shah K, Deakin AH, Kinninmonth AWG (2011) Reduction of blood loss in primary hip arthroplasty with tranexamic acid or fibrin spray. A randomized controlled trial. Acta Orthop 82:660–663
Randelli F, Banci L, Ragone V, Pasevi M, Randelli G (2013) Effectiveneness of fibrin sealant after cementless total hip replacement: a double blind randomized controlled trial. Int J Immunopathol Pharmacol 26:189–197
Alshryda S, Mason J, Sarda P, Nargol A, Cooke N, Ahmad H, Tang S, Logishetty R, Vaghela M, McPartlin L, Hungin APS (2013) Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement. A randomized controlled trial (TRANX-H). J Bone Joint Surg Am 95:1969–1974
Konig G, Hamlin BR, Waters JH (2013) Topical tranexamic acid reduces blood Loss and transfusions rates in total hip and total knee arthroplasty. J Arthroplasty 28:1473–1476
Martin JG, Cassatt KB, Kincaid-Cinnamon KA, Estendorf DS, Garton AS, Lemke JH (2014) Topical administration of tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty 29:889–894
Wei W, Wei B (2014) Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplasty 29:113–116
Yue C, Kang P, Yang P, Xie J, Pei F (2014) Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty 29:2452–2456
Emara WM, Moez KK, Elkhouly AH (2014) Topical versus intravenous tranexamic acid as a blood conservation intervention for reduction of postoperative bleeding in hemiarthroplasty. Anaesth Essays Res 8:48–53
Kang JS, Moon KH, Kim BS, Yang SJ (2016) Topical administration of tranexamic acid in hip arthroplasty. Int Orthop 41:259–263
Acknowledgements
We thank patients who agreed to participate; secretaries, nurses and in general, the staff of Clinical Epidemiology and Publica Health Department-Iberoamerican Cochrane Centre and Tramatology Department of participant centres. To Andrea Cervera for editing the manuscript. Dr. Jordan used this study to develop his PhD at the Universitat Autonoma de Barcelona (Spain). Dr. Mª José Martinez Zapata is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CP15/00116). This project was funded by the Ministry of Health and Social Policy, Spain. Directorate General of Pharmacy and Health Products. “Projects for the translation of the advanced therapeutic application of human medicines, orphans and advanced therapies”. This study also received funding from European Regional Development Fund (FEDER; “A way of making Europe”). Number of project: EC11-341. TRANEXFER Group is also composed by: Julio De Caso: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: JCaso@santpau.cat. Ion Carrera: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: ICarrera@santpau.cat. Angie Millán: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: AMillan@santpau.cat. Mª Carmen Pulido: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: MPulido@santpau.cat. Marius Valera: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: MValera@santpau.cat. Xavier Crusi: Orthopedic and Traumatology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: XCrusi@santpau.cat. José Antonio Fernández Núñez: Anesthesiology Service. Hospital de la Santa Creu I Sant Pau, Barcelona, Spain, e-mail: JFernandezN@santpau.cat. Anna Canalias Bage: Orthopedic and Traumatology Service. Hospital Universitari Mútua Terrassa, Barcelona, Spain, e-mail: anna.canalias@gmail.com. Anna Alavedra: Orthopedic and Traumatology Service. Consorci Hospitalari Parc Taulí, Sabadell, Barcelona, Spain, e-mail: aalavedra@tauli.cat. Margarita Novellas: Anesthesiology Service. Hospital Universitari Terrassa, Barcelona, Spain, e-mail: mnovellas@mutuaterrassa.cat. Francesc Anglès Crespo: Orthopedic and Traumatology Service. Hospital Universitari Mútua de Terrassa, Barcelona, Spain, e-mail: fangles@mutuaterrassa.es. Gerard Urrutia: Public Health and Clinical Epidemiology Service-Iberoamerican Cochrane Centre. IIB Sant Pau. CIBERESP, Barcelona, Spain, e-mail: gurrutia@santpau.cat. Esther Cànovas: Iberoamerican Cochrane Centre-IIB Sant Pau, Barcelona, Spain, e-mail: ecanovas@santpau.cat.
Funding
This project was funded by the Ministry of Health and Social Policy, Spain. Directorate General of Pharmacy and Health Products. “Projects for the translation of the advanced therapeutic application of human medicines, orphans and advanced therapies”. This study also received funding from European Regional Development Fund (FEDER; “A way of making Europe”). Number of project: EC11-341.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national Research Ethics Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
TRANEXFER Group memebers list are given in “Acknowledgement” section.
Rights and permissions
About this article
Cite this article
Jordan, M., Aguilera, X., González, J.C. et al. Prevention of postoperative bleeding in hip fractures treated with prosthetic replacement: efficacy and safety of fibrin sealant and tranexamic acid. A randomised controlled clinical trial (TRANEXFER study). Arch Orthop Trauma Surg 139, 597–604 (2019). https://doi.org/10.1007/s00402-018-3089-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-018-3089-4