Abstract
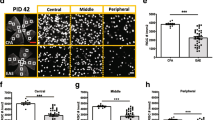
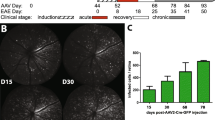
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) characterized by varying degrees of secondary neurodegeneration. Retinal ganglion cells (RGC) are lost in MS in association with optic neuritis but the mechanisms of neuronal injury remain unclear. Complement component C3 has been implicated in retinal and cerebral synaptic pathology that may precede neurodegeneration. Herein, we examined post-mortem MS retinas, and then used a mouse model, experimental autoimmune encephalomyelitis (EAE), to examine the role of C3 in the pathogenesis of RGC loss associated with optic neuritis. First, we show extensive C3 expression in astrocytes (C3+/GFAP+ cells) and significant RGC loss (RBPMS+ cells) in post-mortem retinas from people with MS compared to retinas from non-MS individuals. A patient with progressive MS with a remote history of optic neuritis showed marked reactive astrogliosis with C3 expression in the inner retina extending into deeper layers in the affected eye more than the unaffected eye. To study whether C3 mediates retinal degeneration, we utilized global C3–/– EAE mice and found that they had less RGC loss and partially preserved neurites in the retina compared with C3+/+ EAE mice. C3–/– EAE mice had fewer axonal swellings in the optic nerve, reflecting reduced axonal injury, but had no changes in demyelination or T cell infiltration into the CNS. Using a C3-tdTomato reporter mouse line, we show definitive evidence of C3 expression in astrocytes in the retina and optic nerves of EAE mice. Conditional deletion of C3 in astrocytes showed RGC protection replicating the effects seen in the global knockouts. These data implicate astrocyte C3 expression as a critical mediator of retinal neuronal pathology in EAE and MS, and are consistent with recent studies showing C3 gene variants are associated with faster rates of retinal neurodegeneration in human disease.







Similar content being viewed by others
References
Antel JP, Becher B, Ludwin SK, Prat A, Quintana FJ (2020) Glial cells as regulators of neuroimmune interactions in the central nervous system. J Immunol 204:251–255. https://doi.org/10.4049/jimmunol.1900908
Barbar L, Jain T, Zimmer M, Kruglikov I, Sadick JS, Wang M, et al (2020) CD49f Is a novel marker of functional and reactive human iPSC-derived astrocytes. Neuron https://doi.org/10.1016/j.neuron.2020.05.014
Bebo BF Jr, Dehghani B, Foster S, Kurniawan A, Lopez FJ, Sherman LS (2009) Treatment with selective estrogen receptor modulators regulates myelin specific T-cells and suppresses experimental autoimmune encephalomyelitis. Glia 57:777–790. https://doi.org/10.1002/glia.20805
Brambilla R (2019) The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol 137:757–783. https://doi.org/10.1007/s00401-019-01980-7
Calida DM, Constantinescu C, Purev E, Zhang GX, Ventura ES, Lavi E et al (2001) Cutting edge: C3, a key component of complement activation, is not required for the development of myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis in mice. J Immunol 166:723–726
Elloso MM, Phiel K, Henderson RA, Harris HA, Adelman SJ (2005) Suppression of experimental autoimmune encephalomyelitis using estrogen receptor-selective ligands. J Endocrinol 185:243–252. https://doi.org/10.1677/joe.1.06063
Fitzgerald KC, Kim K, Smith MD, Aston SA, Fioravante N, Rothman AM et al (2019) Early complement genes are associated with visual system degeneration in multiple sclerosis. Brain 142:2722–2736. https://doi.org/10.1093/brain/awz188
Gleichman AJ, Carmichael ST (2020) Glia in neurodegeneration: drivers of disease or along for the ride? Neurobiol Dis 142:104957. https://doi.org/10.1016/j.nbd.2020.104957
Godel C, Kunkel B, Kashani A, Lassmann H, Arumugam M, Krishnamoorthy G (2020) Perturbation of gut microbiota decreases susceptibility but does not modulate ongoing autoimmune neurological disease. J Neuroinflammation 17:79. https://doi.org/10.1186/s12974-020-01766-9
Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R (2010) Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 133:1591–1601. https://doi.org/10.1093/brain/awq080
Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A et al (2009) Deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci 29:1874–1886. https://doi.org/10.1523/jneurosci.3095-08.2009
Guttenplan KA, Stafford BK, El-Danaf RN, Adler DI, Munch AE, Weigel MK et al (2020) Neurotoxic reactive astrocytes drive neuronal death after retinal injury. Cell Rep 31:107776. https://doi.org/10.1016/j.celrep.2020.107776
Hammond JW, Bellizzi MJ, Ware C, Qiu WQ, Saminathan P, Li H et al (2020) Complement-dependent synapse loss and microgliosis in a mouse model of multiple sclerosis. Brain Behav Immun https://doi.org/10.1016/j.bbi.2020.03.004
Hess C, Kemper C (2016) Complement-mediated regulation of metabolism and basic cellular processes. Immunity 45:240–254. https://doi.org/10.1016/j.immuni.2016.08.003
Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S et al (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352:712–716. https://doi.org/10.1126/science.aad8373
Horstmann L, Kuehn S, Pedreiturria X, Haak K, Pfarrer C, Dick HB et al (2016) Microglia response in retina and optic nerve in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol 298:32–41. https://doi.org/10.1016/j.jneuroim.2016.06.008
Ingram G, Loveless S, Howell OW, Hakobyan S, Dancey B, Harris CL et al (2014) Complement activation in multiple sclerosis plaques: an immunohistochemical analysis. Acta Neuropathol Commun 2:53. https://doi.org/10.1186/2051-5960-2-53
Itoh N, Itoh Y, Tassoni A, Ren E, Kaito M, Ohno A et al (2018) Cell-specific and region-specific transcriptomics in the multiple sclerosis model: focus on astrocytes. Proc Natl Acad Sci 115:E302–E309. https://doi.org/10.1073/pnas.1716032115
Jin J, Smith MD, Kersbergen CJ, Kam TI, Viswanathan M, Martin K et al (2019) Glial pathology and retinal neurotoxicity in the anterior visual pathway in experimental autoimmune encephalomyelitis. Acta Neuropathol Commun 7:125. https://doi.org/10.1186/s40478-019-0767-6
Kassa E, Ciulla TA, Hussain RM, Dugel PU (2019) Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opin Biol Ther 19:335–342. https://doi.org/10.1080/14712598.2019.1575358
Katschke KJ Jr, Xi H, Cox C, Truong T, Malato Y, Lee WP et al (2018) Classical and alternative complement activation on photoreceptor outer segments drives monocyte-dependent retinal atrophy. Sci Rep 8:7348. https://doi.org/10.1038/s41598-018-25557-8
Kolev M, West EE, Kunz N, Chauss D, Moseman EA, Rahman J et al (2020) Diapedesis-induced integrin signaling via LFA-1 facilitates tissue immunity by inducing intrinsic complement C3 expression in immune cells. Immunity 52(513–527):e518. https://doi.org/10.1016/j.immuni.2020.02.006
Larabee CM, Desai S, Agasing A, Georgescu C, Wren JD, Axtell RC et al (2016) Loss of Nrf2 exacerbates the visual deficits and optic neuritis elicited by experimental autoimmune encephalomyelitis. Mol Vis 22:1503–1513
Larabee CM, Hu Y, Desai S, Georgescu C, Wren JD, Axtell RC et al (2016) Myelin-specific Th17 cells induce severe relapsing optic neuritis with irreversible loss of retinal ganglion cells in C57BL/6 mice. Mol Vis 22:332–341
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. https://doi.org/10.1038/nature21029
Liu Q, Li H, Yang J, Niu X, Zhao C, Zhao L et al (2017) Valproic acid attenuates inflammation of optic nerve and apoptosis of retinal ganglion cells in a rat model of optic neuritis. Biomed Pharmacother 96:1363–1370. https://doi.org/10.1016/j.biopha.2017.11.066
Lucin KM, Wyss-Coray T (2009) Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64:110–122. https://doi.org/10.1016/j.neuron.2009.08.039
Madore C, Yin Z, Leibowitz J, Butovsky O (2020) Microglia, lifestyle stress, and neurodegeneration. Immunity 52:222–240. https://doi.org/10.1016/j.immuni.2019.12.003
Michailidou I, Naessens DM, Hametner S, Guldenaar W, Kooi EJ, Geurts JJ et al (2017) Complement C3 on microglial clusters in multiple sclerosis occur in chronic but not acute disease: Implication for disease pathogenesis. Glia 65:264–277. https://doi.org/10.1002/glia.23090
Michailidou I, Willems JG, Kooi EJ, van Eden C, Gold SM, Geurts JJ et al (2015) Complement C1q–C3-associated synaptic changes in multiple sclerosis hippocampus. Ann Neurol 77:1007–1026. https://doi.org/10.1002/ana.24398
Nytrova P, Potlukova E, Kemlink D, Woodhall M, Horakova D, Waters P et al (2014) Complement activation in patients with neuromyelitis optica. J Neuroimmunol 274:185–191. https://doi.org/10.1016/j.jneuroim.2014.07.001
Pauly D, Agarwal D, Dana N, Schafer N, Biber J, Wunderlich KA et al (2019) Cell-type-specific complement expression in the healthy and diseased retina. Cell Rep 29(2835–2848):e2834. https://doi.org/10.1016/j.celrep.2019.10.084
Petzold A, Balcer LJ, Calabresi PA, Costello F, Frohman TC, Frohman EM et al (2017) Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol 16:797–812. https://doi.org/10.1016/S1474-4422(17)30278-8
Pluvinage JV, Wyss-Coray T (2020) Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci 21:93–102. https://doi.org/10.1038/s41583-019-0255-9
Ramaglia V, Dubey M, Malpede MA, Petersen N, de Vries SI, Ahmed SM et al (2021) Complement-associated loss of CA2 inhibitory synapses in the demyelinated hippocampus impairs memory. Acta Neuropathol. https://doi.org/10.1007/s00401-021-02338-8
Ramaglia V, Hughes TR, Donev RM, Ruseva MM, Wu X, Huitinga I et al (2012) C3-dependent mechanism of microglial priming relevant to multiple sclerosis. Proc Natl Acad Sci 109:965–970. https://doi.org/10.1073/pnas.1111924109
Reynolds JD, Case LK, Krementsov DN, Raza A, Bartiss R, Teuscher C (2017) Modeling month-season of birth as a risk factor in mouse models of chronic disease: from multiple sclerosis to autoimmune encephalomyelitis. FASEB J 31:2709–2719. https://doi.org/10.1096/fj.201700062
Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE et al (2015) Complement C3-deficient mice fail to display age-related hippocampal decline. J Neurosci 35:13029–13042. https://doi.org/10.1523/JNEUROSCI.1698-15.2015
Silverman SM, Ma W, Wang X, Zhao L, Wong WT (2019) C3- and CR3-dependent microglial clearance protects photoreceptors in retinitis pigmentosa. J Exp Med 216:1925–1943. https://doi.org/10.1084/jem.20190009
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N et al (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–1178. https://doi.org/10.1016/j.cell.2007.10.036
Szalai AJ, Hu X, Adams JE, Barnum SR (2007) Complement in experimental autoimmune encephalomyelitis revisited: C3 is required for development of maximal disease. Mol Immunol 44:3132–3136. https://doi.org/10.1016/j.molimm.2007.02.002
Tassoni A, Farkhondeh V, Itoh Y, Itoh N, Sofroniew MV, Voskuhl RR (2019) The astrocyte transcriptome in EAE optic neuritis shows complement activation and reveals a sex difference in astrocytic C3 expression. Sci Rep 9:10010. https://doi.org/10.1038/s41598-019-46232-6
Tenner AJ, Stevens B, Woodruff TM (2018) New tricks for an ancient system: physiological and pathological roles of complement in the CNS. Mol Immunol 102:3–13. https://doi.org/10.1016/j.molimm.2018.06.264
Watkins LM, Neal JW, Loveless S, Michailidou I, Ramaglia V, Rees MI et al (2016) Complement is activated in progressive multiple sclerosis cortical grey matter lesions. J Neuroinflammation 13:161. https://doi.org/10.1186/s12974-016-0611-x
Werneburg S, Jung J, Kunjamma RB, Ha SK, Luciano NJ, Willis CM et al (2020) Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52(167–182):e167. https://doi.org/10.1016/j.immuni.2019.12.004
Wessels MR, Butko P, Ma M, Warren HB, Lage AL, Carroll MC (1995) Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci U S A 92:11490–11494. https://doi.org/10.1073/pnas.92.25.11490
Wu T, Dejanovic B, Gandham VD, Gogineni A, Edmonds R, Schauer S et al (2019) Complement C3 is activated in human AD brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep 28(2111–2123):e2116. https://doi.org/10.1016/j.celrep.2019.07.060
Yun SP, Kam T-I, Panicker N, Kim S, Oh Y, Park J-S et al (2018) Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 24:931–938. https://doi.org/10.1038/s41591-018-0051-5
Acknowledgements
The authors wish to thank Kyle Martin, Arthur Anthony Reyes, and Hannah-Noelle Lord for assistance with scoring EAE mice. The authors also thank the JHU Ross Flow Cytometry Core for use of a Cytek Aurora with a yellow laser to assess tdT expression.
Funding
This study was funded by; R01 NS041435 (PAC), National MS Society (NMSS) grants RG-1907–34756 (PAC) and SI-2004–36590 (DSR), the Division of Intramural Research of the National Institute of Neurological Disorders and Stroke (NINDS) and the National Heart, Lung, and Blood Institute (NHLBI), NIH (ZIANS003119 to DSR and ZIAHL006223 to CK), Fonds de recherche du Québec—Santé (FRQS)# 270746 to MG, and NIH S10OD026859 to JHU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gharagozloo, M., Smith, M.D., Jin, J. et al. Complement component 3 from astrocytes mediates retinal ganglion cell loss during neuroinflammation. Acta Neuropathol 142, 899–915 (2021). https://doi.org/10.1007/s00401-021-02366-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-021-02366-4