Abstract
This article deals with the modulation of electroosmotic flow (EOF) and transport of ionic species through the parallel plate soft nanochannel. The charged rigid walls of the channel are covered by diffuse polyelectrolyte layer (PEL) which entraps immobile charges. A diffuse distribution of the polymer segment density and charge density is assumed. A nonlinear model based on the Poisson-Nernst-Planck equations coupled with the Darcy-Brinkman equations is adopted. Going beyond the widely employed Debye-H\(\ddot {u}\)ckel linearization, we adopt a sophisticated numerical tool to study the effect of pertinent parameters on the modulation of EOF through the soft nanochannel. Several interesting key features including the flow reversal, occurrence of zero flow rate, and perm selectivity are studied by regulating the charges entrapped within the diffuse PEL and the surface charge distributed along the channel wall. The results indicate that the channel can be cation-selective, anion-selective, and non-selective based on the nature of the charges within the PEL and wall charge. We have also identified the parameter range for validity of the linearized model for the case of step-like PEL.







Similar content being viewed by others
References
Actis P, Vilozny B , Seger RA, Li X, Jejelowo O, Rinaudo M, Pourmand N (2011) Voltage-controlled metal binding on polyelectrolyte-functionalized nanopores. Langmuir 27(10):6528–6533
Ali M, Yameen B, Cervera J, Ramirez P, Neumann R, Ensinger W, Knoll W, Azzaroni O (2010) Layer-by-layer assembly of polyelectrolytes into ionic current rectifying solid-state nanopores: insights from theory and experiment. J Am Chem Soc 132(24):8338–8348
Chanda S, Sinha S, Das S (2014) Streaming potential and electroviscous effects in soft nanochannels: towards designing more efficient nanofluidic electrochemomechanical energy converters. Soft Matter 38:7558–7568
Chen G, Das S (2015) Streaming potential and electroviscous effects in soft nanochannels beyond Debye–Hückel linearization. J Colloid Interface Sci 445:357–363
Chen G, Das S (2017) Massively enhanced electroosmotic transport in nanochannels grafted with end-charged polyelectrolyte brushes. J Phys Chem B 121(14):3130–3141
Ding Z, Fong RB, Long CJ, Stayton PS, Hoffman AS (2001) Size-dependent control of the binding of biotinylated proteins to streptavidin using a polymer shield. Nature 411(6833):59
Duval JFL (2005) Electrokinetics of diffuse soft interfaces. 2. Analysis based on the nonlinear Poisson-Boltzmann equation. Langmuir 21(8):3247–3258
Duval JFL, Ohshima H (2006) Electrophoresis of diffuse soft particles. Langmuir 22(8):3533–3546
Duval JFL, van Leeuwen HP (2004) Electrokinetics of diffuse soft interfaces. 1. Limit of low Donnan potentials. Langmuir 20(23):10324–10336
Duval JFL, Zimmermann R, Cordeiro AL, Rein N, Werner C (2009) Electrokinetics of diffuse soft interfaces. iv. Analysis of streaming current measurements at thermoresponsive thin films. Langmuir 25 (18):10691–10703
Fletcher CAJ (1991) Computational techniques for fluid dynamics, vol 2, 2nd edn. Springer, Berlin
Inglis DW, Goldys EM, Calander NP (2011) Simultaneous concentration and separation of proteins in a nanochannel. Angewandte Chemie Int Edn 50(33):7546–7550
Karnik R, Fan R, Yue M, Li D, Yang P, Majumdar A (2005) Electrostatic control of ions and molecules in nanofluidic transistors. Nano Lett 5(5):943–948
Kim SJ, Ko SH, Kang KH, Han J (2010) Direct seawater desalination by ion concentration polarization. Nat Nanotechnol 5(4):297–301
Kwak R, Kim SJ, Han J (2011) Continuous-flow biomolecule and cell concentrator by ion concentration polarization. Anal Chem 83(19):7348–7355
Leonard BP (1979) A stable and accurate convective modelling procedure based on quadratic upstream interpolation. Comput Methods Appl Mech Eng 19(1):59–98
Machida S, Urano TI, Sano K, Kawata Y, Sunohara K, Sasaki H, Yoshiki M, Mori Y (1995) A chiral director field in the nematic liquid crystal phase induced by a poly (. gamma.-benzyl glutamate) chemical reaction alignment film. Langmuir 11(12):4838–4843
Masliyah JH, Bhattacharjee S (2006) Electrokinetic and colloid transport phenomena. Wiley
Matin MH, Ohshima H (2016) Thermal transport characteristics of combined electroosmotic and pressure driven flow in soft nanofluidics. J Colloid Interf Sci 476:167–176
Matin MH, Ohshima H (2015) Combined electroosmotically and pressure driven flow in soft nanofluidics. J Colloid Interf Sci 460:361–369
Patankar S (1980) Numerical heat transfer and fluid flow. CRC Press
Prakash S, Zambrano HA, Rangharajan KK, Rosenthal-Kim E, Vasquez N, Conlisk AT (2016) Electrokinetic transport of monovalent and divalent cations in silica nanochannels. Microfluid Nanofluid 20(1):1–8
Schmaljohann D (2006) Thermo-and ph-responsive polymers in drug delivery. Adv Drug Delivery Rev 58 (15):1655–1670
Stein D, Kruithof M, Dekker C (2004) Surface-charge-governed ion transport in nanofluidic channels. Phys Rev Lett 93(3):035901
Vlassiouk I, Smirnov S, Siwy Z (2008) Ionic selectivity of single nanochannels. Nano Lett 8(7):1978–1985
Vlassiouk I, Smirnov S, Siwy Z (2008) Nanofluidic ionic diodes. Comparison of analytical and numerical solutions. Acs Nano 2(8):1589–1602
Yameen B, Ali M, Neumann R, Ensinger W, Knoll W, Azzaroni O (2009) Synthetic proton-gated ion channels via single solid-state nanochannels modified with responsive polymer brushes. Nano Lett 9(7):2788–2793
Yang M, Yang X, Wang K, Wang Q, Fan X, Liu W, Liu X, Liu J, Huang J (2015) Tuning transport selectivity of ionic species by phosphoric acid gradient in positively charged nanochannel membranes. Anal Chem 87(3):1544–1551
Yeh L-H, Zhang M, Qian S, Hsu J-P, Tseng S (2012) Ion concentration polarization in polyelectrolyte-modified nanopores. J Phys Chem C 116(15):8672–8677
Zambrano HA, Vásquez N, Wagemann E (2016) Wall embedded electrodes to modify electroosmotic flow in silica nanoslits. Phys Chem Chem Phys 18(2):1202–1211
Zhang Y, Kato S, Anazawa T (2008) A microchannel concentrator controlled by integral thermoresponsive valves. Sens Actuators B 129(1):481–486
Zuo Y, Wang G, Ying Y, Zuo C, Liu Z, Dongmei H, Wang Y (2014) Suppression of electroosmotic flow by polyampholyte brush. Microfluidics Nanofluidics 17(5):923–931
Acknowledgements
One of the authors (P. P. G.) thanks the Science and Engineering Research Board (SERB), Government of India, for providing the financial support through the project grant (File no. YSS/2015/000468).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Appendix
Appendix
We now consider a step-like PEL coating of thickness d1 in which the monomers are uniformly distributed (α = 0). We assume the charge density of the channel walls as well as the immobile charges entrapped within the PEL are low enough so that we can invoke the Debye-H\(\ddot {u}\)ckel approximation to linearize the Poisson-Boltzmann equation. Under these assumptions, the governing equations for the electric potential and the velocity field can be written in nondimensional form as
Here, Q f = (κ s h)2/2 is the scaled charge inside the PEL and d1 = d/h is nominal thickness of the PEL. The above equations are solved by using the following boundary conditions
A symmetry condition is considered along the central line (y = 1/2) of the channel. The continuity conditions along the PEL-electrolyte interface (y = d1) can be expressed as
The closed form solution for the electrostatic potential and axial velocity can be obtained as
The coefficients C i (i = 1, 2,..7) are given by
The expression (A.6) for the axial velocity component involves the scaled parameter β = λ0h, which provides a measure of the permeability of the PEL medium. The hydrodynamic screening length \(\lambda _{0}^{-1}\) is related to the permeability \(\kappa _{p}=\lambda _{0}^{-2}\) of the PEL. Duval and his group ([7,8,9,10]) considered \(\lambda _{0}^{-1}\) in the range 5 to 100 nm for polymer coated channels. For this range of \(\lambda _{0}^{-1}\), the non-dimensional parameter β may vary between 1 and 20 when the channel height h = 100 nm.
The present closed form solutions are different from the expressions obtained by Matin and Ohshima [19, 20]. It may be noted that Matin and Ohshima [19] considered an uncharged wall, whereas in the present study, the channel walls are considered to have a non-zero surface charge density. In addition, Matin and Ohshima [19, 20] obtained the closed form solution by considering a positive sign in the second term of l.h.s of Eq. (A.2). The scaled velocity u c along the center of the channel (y = 1/2) can be obtained as
where the coefficients C3, C4, C5, are C6 are given in (A.7), which involves the scaled surface charge density (σ∗ = σh/ε e ϕ0) of the channel walls and the immobile charge density entrapped within the PEL (Q f = (κ s h)2/2). A detailed discussion on the derivation of these parameters (i.e., σ∗, \(\kappa _{s}^{-1}\)) are already made in “Mathematical model” section. An expression for the axial velocity u c at the central line of the channel for an uncoated channel can be derived from (A.8) by setting the values of the parameters d1 and Q f as zero i.e.,
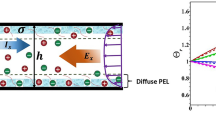
This expression is the same as derived by several authors for EOF in a uncoated slit microchannel (e.g., Masliyah and Bhattacharjee [18]). In Fig. 8a, we have presented the the central line velocity as obtained by (A.8) as a function of Debye-Huckel parameter κh for different values of the PEL charge when β = 1. We found that an increase in κh decreases the value of u c and it approaches a constant as the Debye length becomes sufficiently thinner than h, i.e., κh becomes large. In addition, we have also presented the the central line velocity as a function of the softness parameter β for higher value of κh(= 100). Increase in β decreases the flow penetration across the PEL and it leads to decrease in the magnitude of u c .
Variation of axial velocity at central line of the channel (u c ) with a κh for β = 1 and b β for κh = 100. The results are shown for surface charge density σ = − 1 mC/m2 of the channel wall at different PEL charge density. The nominal thickness of the step-like PEL (α = 0) is considered to be d1 = 0.1
Rights and permissions
About this article
Cite this article
Bag, N., Bhattacharyya, S., Gopmandal, P.P. et al. Electroosmotic flow reversal and ion selectivity in a soft nanochannel. Colloid Polym Sci 296, 849–859 (2018). https://doi.org/10.1007/s00396-018-4293-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4293-z