Abstract
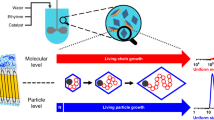
A two-step reprecipitation method for synthesizing semiconducting polymer nanoparticles (NPs) has been developed that overcomes a problem with conventional reprecipitation: the difficulty in controlling the size of smaller particles (<50 nm). First, uniform droplets are prepared by mixing polymer solution with de-ionized (DI) water, and then particles are formed by adding poor solvent. The number of polymers confined to a single droplet is regulated, and the NP size is tuned by adjusting the initial polymer concentration. The synthesis yield and size reproducibility are higher than with conventional reprecipitation. Moreover, a large negative zeta potential (−30 mV) can be obtained, which enables the agglomeration to be effectively controlled without using surfactant. The large zeta potential is apparently due to electrostatic charges caused by friction between the droplets and the DI water during the strong mixing. This method was used to synthesize NPs of poly(3-hexylthiophene) (P3HT) with sizes ranging from 10 to 65 nm. Investigation of the effect of the particle size on various physical properties, including film morphology, absorbance, photoluminescence, and crystallinity, showed that these properties were drastically different at around 50 nm and that they were similar below 30 nm. These results demonstrate the importance of precisely controlling NP size.









Similar content being viewed by others
Abbreviations
- NP:
-
Nanoparticle
- PSC:
-
Polymer solar cell
- BHJ:
-
Bulk-heterojunction
- P3HT:
-
Poly(3-hexylthiophene)
- DI:
-
De-ionized
- M w :
-
Weight average molecular weight
- M n :
-
Number average molecular weight
- AFM:
-
Atomic force microscopy
- SEM:
-
Scanning electron microscopy
- DLS:
-
Dynamic light scattering
- XRD:
-
X-ray diffraction
- PL:
-
Photoluminescence
References
Wu C, Bull B, Szymanski C, Christensen K, McNeill J (2008) Multicolor conjugated polymer dots for biological fluorescence imaging. ACS Nano 2:2415–2423
Nagarjuna G, Baghgar M, Labastide JA, Algaier DD, Barnes MD, Venkataraman D (2012) Tuning aggregation of poly(3-hexylthiophene) within nanoparticles. ACS Nano 6:10750–10758
Richards JJ, Whittle CL, Shao GZ, Pozzo LD (2014) Correlating structure and photocurrent for composite semiconducting nanoparticles with contrast variation small-angle neutron scattering and photoconductive atomic force microscopy. ACS Nano 8:4313–4324
Gehan TS, Bag M, Renna LA, Shen XB, Algaier DD, Lahti PM, Russell TP, Venkataraman D (2014) Multiscale active layer morphologies for organic photovoltaics through self-assembly of nanospheres. Nano Lett 14:5238–5243
Gärtner S, Christmann M, Sankaran S, Röhm H, Prinz EM, Penth F, Pütz A, Tureli AE, Penth B, Baumstummler B, Colsmann A (2014) Eco-friendly fabrication of 4% efficient organic solar cells from surfactant-free P3HT:ICBA nanoparticle dispersions. Adv Mater 26:6653–6657
Millstone JE, Kavulak DFJ, Woo CH, Holcombe TW, Westling EJ, Briseno AL, Toney MF, Fréchet JMJ (2010) Synthesis, properties, and electronic applications of size-controlled poly(3-hexylthiophene) nanoparticles. Langmuir 26:13056–13061
Li G, Shrotriya V, Yao Y, Huang JS, Yang Y (2007) Manipulating regioregular poly(3-hexylthiophene):[6, 6]-phenyl-C61-butyric acid methyl ester blends–route towards high efficiency polymer solar cells. J Mater Chem 17:3126–3140
He YJ, Chen HY, Hou JH, Li YF (2010) Indene-C-60 bisadduct: a new acceptor for high-performance polymer solar cells. J Am Chem Soc 132:1377–1382
Halls JJM, Pichler K, Friend RH, Moratti SC, Holmes AB (1996) Exciton diffusion and dissociation in a poly (p-phenylenevinylene)/C-60 heterojunction photovoltaic cell. Appl Phys Lett 68:3120–3122
Sasaki Y, Kohri M, Kojima T, Taniguchi T, Kishikawa K (2014) Preparation of polymer nanoparticles via phase inversion temperature method using amphiphilic block polymer synthesized by atom transfer radical polymerization. Trans Mat Res Soc Japan 39:125–128
Rao JP, Geckeler KE (2011) Polymer nanoparticles: preparation techniques and size-control parameters. Prog Polym Sci 36:887–913
Pras O, Chaussy D, Stephan O, Rharbi Y, Piette P, Beneventi D (2010) Photoluminescence of 2, 7-poly(9, 9-dialkylfluorene-co-fluorenone) nanoparticles: effect of particle size and inert polymer addition. Langmuir 26:14437–14442
Ulum S, Holmes N, Barr M, Kilcoyne ALD, Gong BB, Zhou XJ, Belcher W, Dastoor P (2013) The role of miscibility in polymer: fullerene nanoparticulate organic photovoltaic devices. Nano Energy 2:897–905
Holmes NP, Burke KB, Sista P, Barr M, Magurudeniya HD, Stefan MC, Kilcoyne ALD, Zhou XJ, Dastoor PC, Belcher WJ (2013) Nano-domain behaviour in P3HT:PCBM nanoparticles, relating material properties to morphological changes. Sol Energy Mater Sol Cells 117:437–445
Hu ZJ, Gesquiere AJ (2009) PCBM concentration dependent morphology of P3HT in composite P3HT/PCBM nanoparticles. Chem Phys Lett 476:51–55
Potai R, Traiphol R (2013) Controlling chain organization and photophysical properties of conjugated polymer nanoparticles prepared by reprecipitation method: the effect of initial solvent. J Colloid Interface Sci 403:58–66
Shimizu H, Yamada M, Wada R, Okabe M (2008) Preparation and characterization of water self-dispersible poly(3-hexylthiophene) particles. Polym J 40:33–36
Lee SH, Lee YB, Park DH, Kim MS, Cho EH, Joo J (2011) Tuning optical properties of poly(3-hexylthiophene) nanoparticles through hydrothermal processing. Sci Technol Adv Mater 12:025002–025007
Kamogawa K, Akatsuka H, Matsumoto M, Yokoyama S, Sakai T, Sakai H, Abe M (2001) Surfactant-free O/W emulsion formation of oleic acid and its esters with ultrasonic dispersion. Colloids Surf A-Physicochem Eng Aspects 180:41–53
Szymanski C, Wu CF, Hooper J, Salazar MA, Perdomo A, Dukes A, McNeill J (2005) Single molecule nanoparticles of the conjugated polymer MEH-PPV, preparation and characterization by near-field scanning optical microscopy. J Phys Chem B 109:8543–8546
Wada M, Sueto T, Takahashi H, Hayashi N, Eitoku A (2008) Study of static electricity in wafer cleaning process. Solid State Phenom 134:263–266
Zhokhavets U, Erb T, Gobsch G, Al-Ibrahim M, Ambacher O (2006) Relation between absorption and crystallinity of poly(3-hexylthiophene)/fullerene films for plastic solar cells. Chem Phys Lett 418:347–350
Fu HB, Yao JN (2001) Size effects on the optical properties of organic nanoparticles. J Am Chem Soc 123:1434–1439
Tagawa N, Masuhara A, Kasai H, Nakanishi H, Oikawa H (2010) Monodispersed and size-controlled diarylethene nanoparticles fabricated by the reprecipitation method. Mol Cryst Liq Cryst 520:245–250
Clark J, Silva C, Friend RH, Spano FC (2007) The role of intermolecular coupling in the photophysics of disordered organic semiconductors: aggregate emission in regioregular polythiophene. Phys Rev Lett 98:206406–206415
Schwarz KN, Farley SB, Smith TA, Ghiggino KP (2015) Charge generation and morphology in P3HT:PCBM nanoparticles prepared by mini-emulsion and reprecipitation methods. Nano 7:19899–19904
Labastide JA, Baghgar M, Dujovne I, Venkatraman BH, Ramsdell DC, Venkataraman D, Barnes MD (2011) Time- and polarization-resolved photoluminescence of individual semicrystalline polythiophene (P3HT) nanoparticles. J Phys Chem Lett 2:2089–2093
Hess BC, Kanner GS, Vardeny Z (1993) Photoexcitations in polythiophene at high pressure. Phys Rev B:Condens Matter 47:1407–1411
Nagai M (2013) Effect of molecular weight and conformation on photoluminescence quantum yield of fluorene/poly(2-methoxy-5-(2′-ethylhexyloxy)-1, 4-phenylene vinylene) (MEH-PPV) copolymers. ECS J Solid State Sci Technol 2:R218–R224
Valeur B (2001) Molecular fluorescence: principles and applications, 1st edn. Wiley-VCH, Weinheim, p. 51
Hillyer MB, Gibb BC (2016) Molecular shape and the hydrophobic effect. Annu Rev Phys Chem 67:307–329
Webster S, Batchelder DN (1996) Absorption, luminescence and Raman spectroscopy of poly (p-phenylene vinylene) at high pressure. Polymer 37:4961–4968
Sirringhaus H (2005) Device physics of solution-processed organic field-effect transistors. Adv Mater 17:2411–2425
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the National Natural Science Foundation of China (21304047), the Natural Science Foundation of Jiangsu Province (13KJB430017), the Research Fund for the Doctoral Program of Higher Education (20133221120015), and the Postgraduate Innovation Foundation of Jiangsu Province (2014, KYZZ_0226).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
( 1079 kb)
Rights and permissions
About this article
Cite this article
Nagai, M., Huang, J., Cui, D. et al. Two-step reprecipitation method with size and zeta potential controllability for synthesizing semiconducting polymer nanoparticles. Colloid Polym Sci 295, 1153–1164 (2017). https://doi.org/10.1007/s00396-017-4097-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4097-6