Abstract
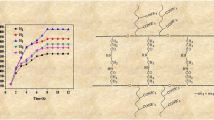
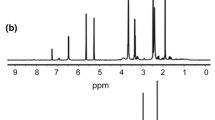
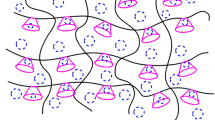
Acrylamide (AAm) and acrylic acid (AAc) were copolymerized in aqueous solution using 2,2-azobis(2-amidinopropane) hydrochloride (V-50) as initiator and N,N′-methylenebisacrylamide (NMBAM) as cross-linking agent to obtain high swelling hydrogels. The effects of AAm/AAc ratio and the amount of cross-linking agent on the swelling properties were studied. It was observed that swelling characteristics of hydrogels are highly affected by the presence of carboxylic groups of AAc units in the hydrogels and by the cross-linking amount in the polymer chains. Hydrogel with 70/30 ratio of AAm/AAc showed the highest swelling degree, until 69.2 g of water/g of dried hydrogel, which represents approximately 7000% of swelling. Swelling kinetic was well represented by a second-order kinetic model for all the AAm/AAc compositions with maximum weight swelling ratio between 20.4 and 82.6 g/g for the hydrogels synthesized using 1% of NMBAM. Diffusion behavior analyses determined that water diffusion into hydrogels followed the anomalous Fickian behavior. The as synthesized hydrogels were used in the removal of Remazol Red 3BS (RR3BS) dye from aqueous solutions, finding that the maximum dye adsorption capacity for an equilibrium aqueous concentration of 130 mg/L of RR3BS was 44.19 mg of RR3BS/g of dried hydrogel with 1% of NMBAM, with an adsorption mechanism well represented by the Langmuir model.








Similar content being viewed by others
References
Xue W, Champ S, Huglin MB (2001) Network and swelling parameters of chemically crosslinked thermoreversible hydrogels. Polymer 42:3665–3669. doi:10.1016/S0032-3861(00)00627-3
Nguyen KT, West JL (2002) Photopolymerizable hydrogels for tissue engineering applications. Biomaterial 23:4307–4314. doi:10.1016/S0142-9612(02)00175-8
Liu MZ, Liang R, Zhan FL, Liu Z, Niu AZ (2006) Synthesis of a slow-release and superabsorbent nitrogen fertilizer and its properties. Polym Adv Technol 47:430–438. doi:10.1002/pat.720
Ali AE, Shawky HA, Abd El Rehim HA, Hegazy EA (2003) Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution. Eur Polym J 39:2337–2344. doi:10.1016/S0014-3057(03)00150-2
Peppas NA, Bures P, Leobandung W, Ichikawa H (2000) Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 50:27–46. doi:10.1016/S0939-6411(00)00090-4
Kopecek J (2007) Hydrogel biomaterials: a smart future? Biomaterials 28:5185–5192. doi:10.1016/j.biomaterials.2007.07.044
Lutolf MP (2009) Biomaterials: spotlight on hydrogels. Nat Mater 8:451–453. doi:10.1038/nmat2458
Maitra J, Shukla VK (2014) Cross-linking in hydrogels—a review. AM J Polym Sci 4:25–31. doi:10.5923/j.ajps.20140402.01
Alemzadeh I, Vossoughi M (2002) Controlled release of paraquat from poly vinyl alcohol hydrogel. Chem Eng Process 41:707–710. doi:10.1016/S0255-2701(01)00190-8
Minhas M, Ahmad M, Ali L, Sohail M (2013) Synthesis of chemically cross-linked polyvinyl alcohol-co-poly (methacrylic acid) hydrogels by copolymerization; a potential graft-polymeric carrier for oral delivery of 5-fluorouracil. J Pharm Sci 21:1–9. doi:10.1186/2008-2231-21-44
Allen SJ, Koumanova B (2005) Decolourisation of water/wastewater using adsorption (review). J U Chem Tech Metal 40:175–192
Khare SK, Panday KK, Srivastava RM, Singh VN (1987) Removal of victoria blue from aqueous solution by fly ash. J Chem Tech Biotechnol 38:99–104. doi:10.1002/jctb.280380206
Karadag E, Saraydin D, Güven O (1996) Interaction of some cationic dyes with acrylamide/itaconic acid hydrogels. J Appl Polym Sci 61:2367–2372. doi:10.1002/(SICI)1097-4628(19960926)61:13<2367::AID-PP16>3.0.CO;2-1
Rodriguez J, Rodrigo MA, Panizza M, Cerisola G (2009) Electrochemical oxidation of acid yellow 1 using diamond anode. J Appl Electrochem 39:2285–2289. doi:10.1007/s10800-009-9880-8
Karadag E, Saraydin D, Güven O (1996) A study on the adsorption of some cationic dyes onto acrylamide/itaconic acid hydrogels. Polym Bull 36:745–752. doi:10.1007/BF00338639
Saraydin D, Karadag E, Güven O (1996) Adsorption of some basic dyes by acrylamide-maleic acid hydrogels. Separation Sci Technol 31:423–434. doi:10.1080/01496399608000705
Saraydin D, Karadag E, Güven O (1996) Behaviors of acrylamide/maleic acid hydrogels in uptake of some cationic dyes from aqueous solutions. Separation Sci Technol 31:2359–2371. doi:10.1080/01496399608001053
Saraydin D, Karadag E, Güven O (2001) Use of superswelling acrylamide/maleic acid hydrogels for monovalent cationic dye adsorption. Appl Polym Sci J 79:1809–1815. doi:10.1002/1097-4628(20010307)79:10<1809::AID-PP90>3.0.CO;2-L
Duran S, Solpan D, Güven O (1999) Synthesis and characterization of acrylamide-acrylic acid hydrogels and adsorption of some textile dyes. Nucl Instr Meth B 151:196–199. doi:10.1016/S0168-583X(99)00151-2
Karadag E, Baris U, Saraydin D (2002) Swelling equilibria and dye adsorption studies of chemically crosslinked superabsorbent acrylamide/maleic acid hydrogels. Eur Polym J 38:2133–2141. doi:10.1016/S0014-3057(02)00117-9
Rojas de Gáscue B, Ramírez M, Prin JL, Torres C, Bejarano L, Villarroel H, Rojas L, Murillo M, Katime I (2010) Hidrogeles de acrilamida/ácido acrilico y de acrilamida/poli(ácido acrilico): estudio de su capacidad de remediación en efluentes industriales. Rev Latinam Metal Mater 30:28–39
Peniche C, Cohen ME, Vazguez B, Roman JS (1997) Water sorption of flexible networks based on 2-hydroxyethyl methacrylate-triethylenglycol dimethacrylate copolymers. Polymer 38:5977–5982. doi:10.1016/S0032-3861(96)01058-0
Peppas NA, Franson NM (1983) The swelling interface number as a criterion for prediction of diffusional solute release mechanisms in swellable polymers. J Polym Sci Pol Phys 21:983–997. doi:10.1002/pol.1983.180210614
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J Control Release 5:37–42. doi:10.1016/0168-3659(87)90035-6
Ostrowska-Czubenko J, Gierszewska M, Pieróg M (2015) pH-responsive hydrogel membranes based on modified chitosan: water transport and kinetics of swelling. J Polym Res 22:153–164. doi:10.1007/s10965-015-0786-3
Karadag E, Saraydin D, Güven O (2004) Water absorbency studies of γ-radiation crosslinked poly (acrylamide-co-2, 3-dihydroxybutanedioic acid) hydrogels. Nucl Instrum Methods Phys Res, Sect B 225:489–496. doi:10.1016/j.nimb.2004.06.010
Murugan R, Mohan S, Bigotto A (1998) FTIR and polarised Raman spectra of acrylamide and polyacrylamide. J Korean Phys Soc 32:505–512
Chitra M, Yang S (2010) Pressure-induced polymerization of acrylic acid: a Raman spectroscopic study. J Phys Chem B 114:9744–9750. doi:10.1021/jp1034757
Guilherme MR, Moia TA, Reis AV, Paulino AT, Rubira AF, Mattoso LHC, Muniz EC, Tambourgi EB (2009) Synthesis and water absorption transport mechanism of a pH-sensitive polymer network structured on vinyl-functionalized pectin. Biomacromolecules 10:190–196. doi:10.1021/bm801250p
Gierszewska-Drużyńska M, Ostrowska-Czubenko J (2012) Mechanism of water diffusion into noncrosslinked and ionically crosslinked chitosan membranes. Journal Progress on Chemistry and Application of Chitin and Its Derivates 17:59–66
Kyzas GZ, Lazaridis NK, Bikiaris DN (2013) Optimization of chitosan and β-cyclodextrin molecularly imprinted polymer synthesis for dye adsorption. Carbohyd Polym 91:198–208. doi:10.1016/j.carbpol.2012.08.016
Ara NJ, Hasan MA, Rahman MA, Salam MA, Salam A, Shafiqul A (2013) Removal of remazol red from textile waste water using treated sawdust—an effective way of effluent treatment. Bangl Pharm J 16:93–98. doi:10.3329/bpj.v16i1.14501
Hafez HS, El-Hag Ali A, Abdel-Mottaleb MSA (2005) Photocatalytic efficiency of titanium dioxide immobilized on PVP/AAc hydrogel membranes: a comparative study for safe disposal of wastewater of remazol red RB-133 textile dye. Int J Photoenergy 7:181–185. doi:10.1155/S1110662X05000279
Ferfera-Harrar H, Aouaz N, Dairi N (2016) Environmental-sensitive chitosan-gpolyacrylamide/carboxymethylcellulose superabsorbent composites for wastewater purification I: synthesis and properties. Polym Bull 73:815–840. doi:10.1007/s00289-015-1521-2
Acknowledgements
This work was supported by the Consejo Nacional de Ciencia y Tecnología (México) for grant no. CB-169444. Author I.D. Antonio-Carmona acknowledges the financial support to PRODEP-SEP for postdoctoral position with the Academic Group of Ingeniería de Procesos Químicos y Ambientales (UASLP-CA-202).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 1718 kb)
Rights and permissions
About this article
Cite this article
Corona-Rivera, M.A., Ovando-Medina, V.M., Bernal-Jacome, L.A. et al. Remazol red dye removal using poly(acrylamide-co-acrylic acid) hydrogels and water absorbency studies. Colloid Polym Sci 295, 227–236 (2017). https://doi.org/10.1007/s00396-016-3996-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3996-2