Abstract
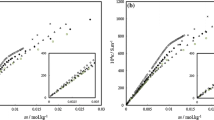
The micro-structural characteristics of sodium dodecyl sulfate (SDS)/benzyl alcohol (BA)/H2O micelle were studied by the methods of theoretical calculation, viscosity, dynamic light scattering, and Raman spectra. The radius of the spherical micelle was about 22.86–35.85 nm. The maximum length of rod-like micelle could reach up to 250.95 nm. In the micelle, the carbon chain of SDS was not completely stretched but still bended. The total chain length of SDS molecule in the micelle appeared to be 2 nm approximately. Both the axis ratio and the shape factor of rod-like micelles increased with the increase of aggregation number. The intrinsic viscosity and relative viscosity of spherical micelles were less than those of the rod-like micelles. The relative viscosity and critical micelle concentration values decreased gradually with the increase of BA content. The second critical micelle concentration of surfactant could also be determined through viscosity method.








Similar content being viewed by others
References
Zhao G, Zhu B (2003) Principles of surfactant action. China Light Industry Press, Beijing
Kaifer AE, Bard AJ (1985) Micellar effects on the reductive electrochemistry of methylviologen. The J Phys Chem 89(22):4876–4880. doi:10.1021/j100268a044
Noritomi H, Tamai S, Saito H, Kato S (2009) Extraction of water miscible organic dyes by reverse micelles of alkyl glucosides. Colloid Polym Sci 287(4):455–459. doi:10.1007/s00396-008-1988-6
Fendler JH (1982) Membrane mimetic chemistry. John Wiley and Sons Ltd., New York
Lu T, Lan Y, Liu C, Huang J, Wang Y (2012) Surface properties, aggregation behavior and micellization thermodynamics of a class of gemini surfactants with ethyl ammonium headgroups. J Colloid Interface Sci 377(1):222–230. doi:10.1016/j.jcis.2012.03.044
Basiruddin SK, Saha A, Pradhan N, Jana NR (2010) Functionalized gold nanorod solution via reverse micelle based polyacrylate coating. Langmuir: ACS J Surf Colloids 26(10):7475–7481. doi:10.1021/la904189a
Lin Y, Alexandridis P (2002) Cosolvent effects on the micellization of an amphiphilic siloxane graft copolymer in aqueous solutions. Langmuir: ACS J Surf Colloids 18(11):4220–4231. doi:10.1021/la011672l
Roberts DW (2002) Application of octanol/water partition coefficients in surfactant science: a quantitative structure–property relationship for micellization of anionic surfactants. Langmuir: ACS J Surf Colloids 18(2):345–352. doi:10.1021/la0108050
Shrestha LK, Shrestha RG, Aramaki K (2011) Intrinsic parameters for the structure control of nonionic reverse micelles in styrene: SAXS and rheometry studies. Langmuir: ACS J Surf Colloids 27(10):5862–5873. doi:10.1021/la200663v
Liu C, Hao J, Wu Z (2010) Phase behavior and rheological properties of salt-free catanionic surfactant mixtures in the presence of bile acids. J Phys Chem B 114(30):9795–9804. doi:10.1021/jp103916a
Jiang Z, Jia K, Liu X, Dong J, Li X (2015) Multiple responsive fluids based on vesicle to wormlike micelle transitions by single-tailed pyrrolidone surfactants. Langmuir: ACS J Surf Colloids 31(43):11760–11768. doi:10.1021/acs.langmuir.5b02312
Jiang LX, Huang JB, Bahramian A, Li PX, Thomas RK, Penfold J (2012) Surface behavior, aggregation and phase separation of aqueous mixtures of dodecyl trimethylammonium bromide and sodium oligoarene sulfonates: the transition to polyelectrolyte/surfactant behavior. Langmuir: ACS J Surf Colloids 28(1):327–338. doi:10.1021/la2040938
Lorente E, Rodríguez A, Aicart E, Junquera E (2007) Non-ionic and cationic micelle nanostructures as drug solubilization vehicles: spectrofluorimetric and electrochemical studies. Colloid Polym Sci 285(12):1321–1329. doi:10.1007/s00396-007-1689-6
Zhang L, Gao L, Liu Q, Yang F, Fang Y (2012) A novel surfactant-like fluorophore and its probing ability to the aggregation of amphiphilic compounds. J Photochem Photobiol A Chem 245:58–65. doi:10.1016/j.jphotochem.2012.07.001
Wang X, Wang J, Wang Y, Ye J, Yan H, Thomas RK (2005) Properties of mixed micelles of cationic gemini surfactants and nonionic surfactant triton X-100: effects of the surfactant composition and the spacer length. J Colloid Interface Sci 286(2):739–746. doi:10.1016/j.jcis.2005.01.084
Gao N, Dong J, Zhang G, Zhou X, Eastoe J, Mutch KJ, Heenan RK (2007) Surface and micelle properties of novel multi-dentate surfactants. J Colloid Interface Sci 314(2):707–711. doi:10.1016/j.jcis.2007.06.001
Joshi JV, Aswal VK, Goyal PS (2007) Effect of sodium salicylate on the structure of micelles of different hydrocarbon chain lengths. Phys B Condens Matter 391(1):65–71. doi:10.1016/j.physb.2006.08.050
Sharma VK, Mitra S, Garcia Sakai V, Mukhopadhyay R (2012) Dynamical features in cationic micelles of varied chain length. J Phys Chem B 116(30):9007–9015. doi:10.1021/jp304841a
Bergstrom LM, Garamus VM (2012) Structural behaviour of mixed cationic surfactant micelles: a small-angle neutron scattering study. J Colloid Interface Sci 381(1):89–99. doi:10.1016/j.jcis.2012.05.015
López-Cervantes JL, Gracia-Fadrique J, Calvo E, Amigo A (2013) Surface tensions, densities, and speeds of sound for aqueous solutions of lauryl ether ethoxylates. Fluid Phase Equilib 356:193–200. doi:10.1016/j.fluid.2013.07.031
Bielawska M, Chodzińska A, Jańczuk B, Zdziennicka A (2013) Determination of CTAB CMC in mixed water + short-chain alcohol solvent by surface tension, conductivity, density and viscosity measurements. Colloids Surf A Physicochem Eng Asp 424:81–88. doi:10.1016/j.colsurfa.2013.02.017
Zdziennicka A, Szymczyk K, Krawczyk J, Jańczuk B (2012) Critical micelle concentration of some surfactants and thermodynamic parameters of their micellization. Fluid Phase Equilib 322–323:126–134. doi:10.1016/j.fluid.2012.03.018
Mitsionis AI, Vaimakis TC (2012) Estimation of AOT and SDS CMC in a methanol using conductometry, viscometry and pyrene fluorescence spectroscopy methods. Chem Phys Lett 547:110–113. doi:10.1016/j.cplett.2012.07.059
Ghosh S, Krishnan A, Das PK, Ramakrishnan S (2003) Determination of critical micelle concentration by hyper-Rayleigh scattering. J Am Chem Soc 125:1602–1606. doi:10.1021/ja029070r
Cifuentes A, Bernal JL, Diez-Masa JC (1997) Determination of critical micelle concentration values using capillary electrophoresis instrumentation. Anal Chem 69:4271–4274. doi:10.1021/ac970696n
Stanley FE, Warner AM, Schneiderman E, Stalcup AM (2009) Rapid determination of surfactant critical micelle concentrations using pressure-driven flow with capillary electrophoresis instrumentation. J Chromatogr A 1216(47):8431–8434. doi:10.1016/j.chroma.2009.09.026
Song Y, Sun R, Zhao K, Pan X, Zhou H, Li D (2015) An induction current method for determining the critical micelle concentration and the polarity of surfactants. Colloid Polym Sci 293(5):1525–1534. doi:10.1007/s00396-015-3536-5
Tan CH, Huang ZJ, Huang XG (2010) Rapid determination of surfactant critical micelle concentration in aqueous solutions using fiber-optic refractive index sensing. Anal Biochem 401(1):144–147. doi:10.1016/j.ab.2010.02.021
Savaroglu G, Yurt A (2011) Determination of the second critical micelle concentration of benzyldimethyltridecylazanium chloride in aqueous solution by acoustic and conductometric measurements. J Chem Thermodyn 43(10):1552–1556. doi:10.1016/j.jct.2011.05.011
González-Pérez A, Czapkiewicz J, Prieto G, Rodríguez JR (2003) Second critical micelle concentration of dodecyldimethylbenzylammonium chloride in aqueous solution at 25°C. Colloid Polym Sci 281(12):1191–1195. doi:10.1007/s00396-003-0905-2
Asakawa T, Mouri M, Miyagishi S, Nishida M (1989) Probe methods for the second cmc of fluorocarbon and hydrocarbon surfactant mixtures. Langmuir: ACS J Surf Colloids 5(2):343–348. doi:10.1021/la00086a009
Mu J, Li G, Zhang W, Wang Z (2001) Determination of the second CMCs of dodecyl polyoxyethylene polyoxypropylene ether by the methods of cloud point, fluorescence, and viscosity. Colloids Surf A Physicochem Eng Asp 194:1–6. doi:10.1016/S0927-7757(01)00782-8
Kodama M, Kubota Y, Miura M (1972) The second cmc of the aqueous solution of sodium dodecyl sulfate. III. Light scattering. Bull Chem Soc Jpn 45(9):2953–2955. doi:10.1246/bcsj.45.2953
Tianqing L, Rong G, Genping S (2007) Determination of the diffusion coefficient for SDS, micelle with different shape and the effects of ethanol by cyclic voltammetry without probes. J Dispers Sci Technol 17(5):509–526. doi:10.1080/01932699608943520
Guo R, Tianqing L, Weili Y (1999) Phase behavior and structure of the sodium dodecyl sulfate/benzyl alcohol/water system. Langmuir: ACS J Surf Colloids 15(2):624–630. doi:10.1021/la9711488
Priyanto S, Mansoori GA, Suwono A (2001) Measurement of property relationships of nano-structure micelles and coacervates of asphaltene in a pure solvent. Chem Eng Sci 56(24):6933–6939. doi:10.1016/S0009-2509(01)00337-2
Romani AP, Machado AE, Hioka N, Severino D, Baptista MS, Codognoto L, Rodrigues MR, de Oliveira HP (2009) Spectrofluorimetric determination of second critical micellar concentration of SDS and SDS/Brij 30 systems. J Fluoresc 19(2):327–332. doi:10.1007/s10895-008-0420-4
Mandal AB, Balachandran UN, Ramaswamy D (1988) Determination of the critical micelle concentration of surfactants and the partition coefficient of an electrochemical probe by using cyclic voltammetry. Langmuir 4(3):736–739. doi:10.1021/la00081a041
Hiemenz PC, Rajagopalan R (1997) Principles of colloid and surface chemistry, 3rd edn. Marcel Dekker, Inc., New York
Bahadur P, Pandya K (1992) Aggregation behavior of Pluronic P-94 in water. Langmuir: ACS J Surf Colloids 8(11):2666–2670. doi:10.1021/la00047a016
Bahadur P, Pandya K, Almgren M, Li P, Stilbs P (1993) Effect of inorganic salts on the micellar behaviour of ethylene oxide-propylene oxide block copolymers in aqueous solution. Colloid Polym Sci 271(7):657–667. doi:10.1007/BF00652828
Zhou Z, Wu P, Xiao Z (1985) Viscometric study of sphere-to-rod transition of aqueous micellar solutions. Acta Phys -Chim Sin 1(4):340–348
Tanford C (1961) Physical chemistry of macromolecules. Wiley, New York
Tanford C (1980) The hydrophobic effect: formation of micelles and biological membranes, 2nd edn. Wiley-Interscience
Dolenko TA, Burikov SA, Dolenko SA, Efitorov AO, Mirgorod YA (2015) Raman spectroscopy of micellization-induced liquid–liquid fluctuations in sodium dodecyl sulfate aqueous solutions. J Mol Liq 204:44–49. doi:10.1016/j.molliq.2015.01.021
Zhu Z, Gu R, Lu T (1998) The application of Raman spectroscopy in chemistry. Northeastern University Press, Shenyang
Miranda AM, Castilho-Almeida EW, Martins Ferreira EH, Moreira GF, Achete CA, Armond RASZ, Dos Santos HF, Jorio A (2014) Line shape analysis of the Raman spectra from pure and mixed biofuels esters compounds. Fuel 115:118–125. doi:10.1016/j.fuel.2013.06.038
Acknowledgments
This work was supported by the National Natural Science Foundation of China (20573091) and Key Laboratory Research Funds of Environmental Materials and Environmental Engineering of Jiangsu Province (K14021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Guo, J., Liu, T., Wang, Y. et al. Determination and calculation of micro-structural parameters of SDS/BA/H2O micelle. Colloid Polym Sci 294, 1289–1295 (2016). https://doi.org/10.1007/s00396-016-3884-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3884-9