Abstract
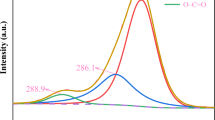
In this paper, we reported a novel non-aqueous electrorheological (ER) fluid structured by TiO2 nano-particle-modified poly (methyl methacrylate) (PMMA/TiO2) dispersed in low viscosity Isopar L and its electrorheological behaviors were researched. Titanium dioxide nano-particles modified with poly (methyl methacrylate) were prepared via in situ polymerization and characterized by Fourier transform infrared, thermogravimetry, and transmission electron microscopy. The thickness of the cladding layer of nano-titanium dioxide surface was about 2∼3 nm, and the cladding rate was 1.437 %. A non-aqueous electrorheological (ER) fluid was constituted by PMMA/TiO2 particles dispersed in Isopar L. The influence of the electric field intensity, the mass concentration of the PMMA/TiO2, and the temperature on the electrorheological properties of ER fluid were discussed, respectively. The research results showed that the ER fluid performed a well rheological property when an external electric field was applied, and with the increase of the electric field intensity, from 0 to 4.5 kV/mm, the shear stress was increased from about 3 to 30 Pa. Meanwhile, the electrorheological effect and shear stress were also strengthened with temperature elevated, and the mass concentration of PMMA/TiO2 particles increased in dispersed system, respectively.








Similar content being viewed by others
References
Whittle M, Blullough WA (1992) The structure of smart fluids. Nature 358:373
Bolck H, Kelly JP (1988) Electro-rheology. J Phys D Appl Phys 21:1661–1677
Halsay TC (1992) Electrorheological fluid. Science 258:761–766
Winslow WM (1949) Induced fibration of suspensions. J Appl Phys 20:1137
Hao T (2001) Electrorheological fluids. Adv Mater 13:1847–1857
Jiang J, Tian Y, Meng Y (2011) Structure parameter of electrorheological fluids in shear flow. Langmuir 27:5814–5823
Hu Y (1998) Effects of an Inner Helmholtz layer on the dielectric dispersion of colloidal suspensions. Langmuir 14:271
Zhang YL, Lu KQ, Rao GH (2002) Electrorheological fluid with an extraordinarily high yield stress. Appl Phys Lett 80:888–890
Méheust Y, Parmar KPS, Schjelderupsen B (2011) The electrorheology of suspensions of Na-fluorohectorite clay in silicone oil. J Rheol 55:809–833
Lu KQ, Wen WJ, Li CX (1995) Frequency dependence of electrorheological fluids in an ac electric field. Phys Rev E 52:6329–6332
Block H, Kelly JP, Qin A (1990) Materials and mechanisms in electrorheology. Langmuir 6:6–14
Kanu RC, Shaw MT (1998) Enhanced electrorheological fluids using anisotropic particles. J Rheol 42:657–670
Tian Y, Meng Y, Wen S (2003) Particulate volume effect in suspensions with strong electrorheological response. Mater Lett 57:2807–2811
Tan ZJ, Zou XW, Zhang WB (1999) Influences of the size and dielectric properties of particles on electrorheological response. Phys Rev E 59:3177–3181
Sequeira V, Hill D (1998) Particle suspensions in liquid crystalline media: rheology, structure, and dynamic interactions. J Rheol 42:203–213
Wu CW, Conrad H (1997) Dielectric and conduction effects in non-Ohmic electrorheological fluids. Phys Rev E 56:5789–5797
Lan YC, Men SQ, Zhao XP (1998) The dependence of particle permittivity on the shear stress of electrorheological fluids. Appl Phys Lett 72:653–655
Uejima H (1972) Dielectric mechanism and rheological properties of electro-fluids. Jpn J Appl Phys 11:319–326
Conrad H, Li Y, Chen Y (1995) The temperature dependence of the electrorheology and related electrical properties of corn starch/corn oil suspensions. J Rheol 39:1041–1057
Erol O, Tejada MR (2013) Effect of surface properties on the electrorheological response of hematite/silicone oil dispersions. J Colloid Interface Sci 392:75–82
Zhao XP, Yin JB (2002) Preparation and electrorheological characteristics of rare-earth-doped TiO2 suspensions. Chem Mater 14:2258–2263
Cao JG, Shen M, Zhou LW (2006) Preparation and electrorheological properties of triethanolamine-modified TiO2. J Solid State Chem 179:1565–1568
Xiang LQ, Zhao XP (2006) Preparation of montmorillonite/titania nanocomposite and enhanced electrorheological activity. J Colloid Interface Sci 1:131–140
Haroun AA, Youssef AM (2011) Synthesis and electrical conductivity evaluation of novel hybrid poly (methyl methacrylate)/titanium dioxide nanowires. Synth Met 161:2063–2069
Ivanova T, Harizanova A (2001) Characterization of TiO2-MnO oxides prepared by sol-gel method. Solid State Ionics 138:227–232
Kor YK, See H (2010) The electrorheological response of elongated particles. Rheol Acta 49:741–756
Klingenberg DJ, van Swol F, Zukoski CF (1989) Dynamic simulation of electrorheological suspensions. J Chem Phys 91:7888–7895
Deinega YF, Vinogradov GV (1984) Electric fields in the rheology of disperse systems. Rheol Acta 23:636–651
Kim YD, Kim JH (2008) Synthesis of polypyrrole-polycaprolactone composites by emulsion polymerization and the electrorheological behavior of their suspensions. Colloid Polym Sci 286:631–637
Klass DL, Martinek TW (1967) Electroviscous fluids. I. rheological properties. J Appl Phys 38:67–74
Conrad H, Sprecher AF (1991) Characteristics and mechanisms of electrorheological fluids. J Stat Phys 64:1073–1091
Cho MS, Cho YH, Choi HJ (2003) Synthesis and electrorheological characteristics of polyaniline-coated poly (methyl methacrylate) microsphere: size effect. Langmuir 19:5875–5881
Hao T, Kawai A, Ikazaki F (1998) Mechanism of the electrorheological effect: evidence from the conductive, dielectric, and surface characteristics of water-free electrorheological fluids. Langmuir 14:1256–1262
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21102098) and the National High Technology Research and Development Program of China (863 Program, No. 2013AA032003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Y., Li, X., Wang, S. et al. Preparation of titanium dioxide nano-particles modified with poly (methyl methacrylate) and its electrorheological characteristics in Isopar L. Colloid Polym Sci 293, 473–479 (2015). https://doi.org/10.1007/s00396-014-3434-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3434-2