Abstract
Cardiovascular diseases and cancer are the leading causes of death in the Western world and share common risk factors. Reduced cardiorespiratory fitness (CRF) is a major determinant of cardiovascular morbidity and cancer survival. In this review we discuss cancer- induced disturbances of parenchymal, cellular, and mitochondrial function, which limit CRF and may be antagonized and attenuated through exercise training. We show the impact of CRF on cancer survival and its attenuating effects on cardiotoxicity of cancer-related treatment. Tailored exercise programs are not yet available for each tumor entity as several trials were performed in heterogeneous populations without adequate cardiopulmonary exercise testing (CPET) prior to exercise prescription and with a wide variation of exercise modalities. There is emerging evidence that exercise may be a crucial pillar in cancer treatment and a tool to mitigate cardiotoxic treatment effects. We discuss modalities of aerobic exercise and resistance training and their potential to improve CRF in cancer patients and provide an example of a periodization model for exercise training in cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular diseases and cancer are the leading causes of death in the Western world and share common risk factors, such as smoking, arterial hypertension, diabetes, sedentary lifestyle, aging, unbalanced diet, and alcohol consumption [122]. In 2022, almost 2 million new cancer cases and more than 600.000 cancer deaths were registered in the USA, including approximately 350 deaths per day from lung cancer, which is the leading cause of cancer death [182]. In 2020 breast cancer was the world´s leading cause of cancer diagnosis with 2.26 million cases [216]. Apart from interventions to reduce common risk factors, the World Health Organization recommends physical activity on a regular basis [216]. As the COVID-19 pandemic has set back cancer research and treatment progress, the Lancet Oncology European Groundshot Commission was founded, which created an action plan aiming to achieve a 10-year survival for 70% of all European cancer patients by 2035 [109]. In this review, we discuss the cardiovascular risk of cancer patients as well as cancer treatment-related cardiotoxicity. We highlight limitations in cardiorespiratory fitness (CRF) induced by parenchymal, cellular, and mitochondrial dysfunction. Mechanisms of cancer-related disruption of signaling and metabolic pathways, which culminate in impaired energy expenditure, are illustrated. Cardiopulmonary exercise testing (CPET) may present a promising diagnostic approach to tailor exercise programs designed to antagonize those effects on a molecular and macroscopic level. We performed a systematic research in PubMed® using the terms “exercise” AND “cancer” as well as “sports” AND cancer “ to search for clinical trials, randomized controlled trials, and meta-analyses. Clinical, translational, and basic science articles were screened and those with objective documentation of exercise intervention or physical activity (PA) were included in this review.
Cardiovascular risk and cardiotoxicity in cancer patients
Patients suffering from cancer bear a higher risk for the development of cardiovascular diseases compared to age-matched controls [122]. Although treatment options of various cancer types have improved over the last decade, specific agents used in cancer therapy increase the probability to develop cardiovascular diseases, arterial hypertension, hypercholesterolemia, diabetes, and may increase body weight [39, 47] as well as the incidence of cardiovascular adverse events [141].
Cancer therapies, especially breast cancer treatment with anthracyclines, increase the risk to develop heart failure [2]. Breast cancer survivors display a higher risk of cardiovascular death eight years after diagnosis [162]. Early initiation of heart failure therapy in breast cancer patients with cardiotoxicity due to anthracycline therapy has been shown to be able to recover left ventricular function [147].
Chemotherapy as well as antiangiogenic drugs have been associated with an increased risk of adverse cardiac events: Bevacizumab has been associated with hypertension, anthracyclines with heart failure and left ventricular dysfunction, taxanes with arrhythmias, and antimetabolites with thromboemboli and spasms [122, 141]. Certain drugs predispose to pulmonary hypertension, such as tyrosine kinase inhibitors and alkylating agents, or increase the risk of ischemic events and arterial hypertension (bevacizumab), which can increase the risk to develop heart failure [79, 194, 195]. Immune checkpoint inhibition targeting programmed cell death 1 disrupts cardiac immune function and may lead to immune mediated myocarditis [102, 133, 137], while chimeric antigen receptor—T cell therapy can trigger cytokine release syndrome, which facilitates cardiovascular events [191, 192]. Specific ECG screening prior to chemotherapy [158] as well as biomarkers such as NTproBNP and troponin and approaches in echocardiography have been regarded as valuable markers to detect apparent and sub-clinical cancer therapy-related cardiotoxicity [73, 134,135,136]. Similar data are available for nuclear and molecular imaging techniques [93, 190]. In more compromised patients with advanced cancer stage the self-reported ability to walk for 4 minutes and wash oneself were independent predictors of survival [11].
Cancer patients are a high-risk population, in which long-term mortality following cardiological interventions is higher compared to non-cancer patients [89, 116, 143, 187] and procedures may even be denied due to shortened life expectancy [43, 116]. Low left ventricular mass and reduced handgrip strength reflect lower functional status and increased all-cause mortality in patients with cancer without manifest cardiovascular disease [65, 114]. Due to the complexity of these patients, multi-disciplinary cardio-oncology teams should be established to optimize preventive and therapeutic measures [196] and further trials to investigate benefits of cardio-protective drugs in cancer patients are still warranted [193].
Although cardio-oncology has been introduced as a sub-discipline to investigate cardiotoxic effects of cancer itself and associated therapies [113, 164], the deleterious consequences of tumor therapy extend far beyond the cardiovascular all the way to the skeletal system [172, 177, 188]. Alterations in oxygen uptake, delivery, extraction, and utilization can occur from mouth to mitochondrion “ leading to a variation of clinical symptoms (Fig. 1)”. Molecular adaptations to cancer and cancer-related therapy impair CRF.
Differential molecular regulation of cancer cells
Mitochondria are intracellular organelles which are involved in energy production through cleavage of phosphate from adenosine triphosphate and receive substrates from the cytoplasm to drive fatty acid oxidation, the tricarboxylic acid cycle, and the electron transport chain. They also contribute to synthesize amino acids, nucleotides, heme, lipids, NADPH, and modulate reactive oxygen species (ROS) for their own metabolic defense [223]. Mitochondrial chaperones, such as heat shock protein 60, assist unfolded proteins to reach their mature configuration [77]. Due to their central role in each cell`s energy production, mutations, be it cancer-induced or inherited, result in severe disturbance of cell function and culminate in systemic diseases, which especially affect the heart, nervous system, and muscle cell [45].
Cancerogenesis is a complex process, which can be induced by oncogenic driver mutations or loss-of-function of tumor suppressor genes [117]. It may also be linked to cell disturbances and affection of mitochondrial integrity through mitochondrial DNA mutations [55, 185], dysregulation of oxidative phosphorylation (OXPHOS) and ROS production [111], as well as impairment of mitochondrial repair and diversification through interference with mitochondrial fission, apoptosis, and mitophagy [91, 166]. The latter is a form of autophagy applied to eliminate non-functional components [119]. It is noteworthy that some tumor cells display a differential regulation of intracellular signaling, depending on whether they are fast or slowly dividing cancer cells. Intracellular metabolism may vary between cancer stem cells and bulk cancer cells [119] (for an overview see Fig. 2). For instance, many tumor cells display a high capacity of OXPHOS, while some mutations, such as Ras transformation, lead to a reduction of mitochondrial capacity [54].
Another mechanism of cancerogenesis and metastasis is imbalance of intercellular signaling and communication between mitochondria of different tissues, which is facilitated by mitokines, such as growth differentiation factor 15 and fibroblast growth factor 21 as well as humanin, a mitochondrial-derived peptide. Disturbances of mitokine signaling contribute to cancerogenesis and heart failure development and may be partly mitigated by regular exercise training [25].
Anticancer therapy usually attempts to inhibit specific pathways. Potential anticancer treatment is being investigated by interfering with mitochondrial ROS production [222], inhibition of autophagy [215], or enzymes of the tricarboxylic acid cycle [28]. Metformin, a complex I inhibitor, may show properties counteracting tumor growth in such tumors which specifically depend on OXPHOS, such as Ras-driven pancreatic cancer subtypes [120]. As tumor cells may partly display a differential regulation of cellular pathways (Fig. 2), inhibition of a single molecule or pathway may not cure cancer without negatively affecting healthy tissue. Pleiotropic effects of cancer treatment are needed to eliminate cancer and reduce side effects to a minimum. Anticancer treatment between 12 and 26 weeks can lead to significant decrease in CRF of up to 26% compared to a natural decline of 10% per decade of normal aging [48]. Cardiotoxic anthracycline therapy for instance increased the expression of the cellular senescence marker cyclin-dependent kinase inhibitor 2A which remained elevated up to one year in patients treated with primary breast cancer; this increase corresponds to almost 15 years of chronological aging [171]. By exerting a differential effect on cellular pathways, exercise may be a potential tool to stabilize CRF, antagonize tumor growth, improve intercellular signaling, and reduce systemic side effects.
Molecular mechanisms of exercise
Growing evidence confirms the benefits of exercise on CRF in cancer patients [122]. Knowledge on molecular mechanisms of exercise in physiological skeletal and myocardial cells is increasing, but the molecular impact of exercise on cancer cells is insufficiently understood.
Anthracyclines, a common group of therapeutics in breast cancer, exert diverse effects on cancer cells including DNA damage and adduct formation, interferences with topoisomerase II, mitochondrial and cell membrane integrity, as well as ROS disturbances and initiation of apoptosis [32, 129]. These effects, however, are not only restricted to cancer cells, but also affect skeletal and myocardial muscle cells [129]. Exercise as a “pleiotropic drug” not only aims to reduce cardiovascular events but also strives to attenuate or prevent toxic effects of cancer therapy on the myocardium, skeletal muscle, and the endothelium. A few daily minutes of vigorous exercise for 3 days demonstrated a protective effect against cellular stress in post-menopausal women, in whom telomere length was longer compared to sedentary controls suggesting a protective effect against cell senescence and morbidity [161].
Effects of exercise displayed different results in animal cancer models: Chronic exercise demonstrated positive effects in a rat model before doxorubicin treatment and led to a better preservation of left ventricular function and greater cardiac expression of heat shock protein 72 [32], which increases cellular stress tolerance. A detrimental effect on tumorigenesis could be shown in a p53 deficient mouse training model [35]: Mice undergoing wheel running displayed a higher rate of mammary cancer than sedentary controls, despite positive effects of exercise on weight and fat content. Although no data are available on exercise intensity in these mice, these findings raised the question about a potential harm of exercise in patients prone to cancer development and a deficient mechanism to induce cell apoptosis. These findings were antagonized by a study taking serum from exercising patients with prostate cancer, in whom tumor apoptosis was induced by increasing cellular p53 content as compared to sedentary controls [115].
Exercise leads to sympathetic activation and release of catecholamines, such as epinephrine, which, if released excessively, may have a negative effect on the cardiovascular system. Exercise-induced, dosed release of epinephrine in five mouse tumor models led to the activation of cytotoxic natural killer cells and culminated in a 60% reduction of tumor growth [153].
Disturbances of cell–cell adhesion molecules, such as cadherins, can facilitate cancer metastasis [92]. Intestinal tumor growth was inhibited in two different mouse models undergoing voluntary wheel running leading to a reduction of the ratio of insulin-like growth factor 1 and its binding protein 3, while higher ratios were observed in colorectal cancers [88]. Nuclear beta-catenin, a main driver of intestinal tumorigenesis, was lowered and E-cadherin, an important cell adhesion molecule, was increased, which may be a mechanistic explanation to lower the rate of metastases [88].
Necrotic areas in bulk tumor cells may not be easily accessible to cancer therapy [129]. Aerobic training induces vascular wall shear stress and induces vascular endothelial growth factor release into the circulation, which triggers nitric oxide biosynthesis and results in vasodilatation and an increase of perfusion [123]. Mechanistically, an increase of perfusion, which is gained with exercise training, also leads to improved drug delivery to tumor cells [86].
Intracellular effects of AET have mainly been studied in myocardial and skeletal muscle cells [6, 123, 224]. Intracellular signaling is modified through AET by downregulation of protein kinase B, phospatidylinositol-3-kinase and the mammalian target of rapamycin, which are also targeted by chemotherapy [7].
Regular exercise increases mitochondrial function of skeletal and myocardial muscle cells by improving the activity of enzymes, such as cytochrome oxidase, succinate dehydrogenase, and succinate oxidase, and facilitates electron transport capacity to produce adenosine triphosphate [75]. Mitochondrial transcription is, among others, regulated by transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) [174]. Exercise induces PGC-1α dependent signaling pathways leading to the activation of calcium/calmodulin-dependent protein kinase [148], phosphorylation of AMP-activated protein kinase [81, 217], and activation of p38mitogen-activated protein kinase, which responds to ROS [81]. PGC-1α also interacts with nuclear respiratory factor 1/2, which facilitates activation of mitochondrial transcription factor A to activate mitochondrial proteins [60].
Exercise improves mitochondrial respiratory capacity, OXPHOS [160] and modulates ROS activity in skeletal, endothelial, and myocardial muscle cells [6, 76]. Importantly, exercise stimuli increase mitochondrial content independent of muscle fibre type [121]. Recruitment of type I fibres is best achieved with endurance training intensities below 40% of VO2peak, while type II fibres require more intense stimuli to optimize neuromuscular transmission [167]. Resistance training (RT) also enhances mitochondrial gene expression and mass [160]. Similar to low-intensity endurance training, high-intensity training (HIIT) triggers PGC-1α-dependent increase of mitochondrial synthesis, but achieves higher calcium release and can produce around 30% increase of mitochondrial content in a shorter period of time [123]. As early as six hours following exercise, mitophagy is enhanced by an increase of the adenosine mono-to-triphosphate ratio, which leads to activation of adenosine monophosphate- activated protein kinase and its downstream target Unc-51 like autophagy activating kinase 1. The mammalian target of rapamycin complex 1, a known suppressor of this activation, is inhibited [106].
Acute exercise leads to a localization of Parkin to skeletal muscle cell mitochondria as well as an increase in mitophagy flux, both processes which are coupled to PGC-1α [199]. Chronic exercise triggers activation of transcription factor EB as well as lysosomal markers mucolipin, Cathepsin D, and lysosomal associated membrane proteins 1 and 2 [95]. Thus, exercise-induced activation of lysosomal and autophagy-related genes facilitates cell organelles, such as mitochondria, to clear cell debris and uphold the necessary intracellular milieu to sustain performance (Fig. 3).
Selected exercise-induced molecular mechanisms in skeletal muscle cells. Exercise activates the central player (in bold) peroxisome proliferator-activated receptor gamma co-activator-1alpha (PGC-1α), which in turn facilitates the upregulation of calcium–calmodulin-dependent kinase (caMK) and 5`-adenomonophosphate-activated protein kinase (AMPK). Furthermore, p38 mitogen-activated protein kinase (p38 MAPK) is activated leading to an increase of mitochondrial synthesis. To stimulate autophagy of mitochondria and lysosomes, AMPK triggers activation of Unc-51 like autophagy activating kinase 1 (ULK 1). In addition, PGC-1α stimulates nuclear respiratory factor (NRF 1/2), which activates mitochondrial transcription factor A (tfam) and also results in enhanced mitochondrial synthesis. Furthermore, exercise facilitates modulation of reactive oxygen species (ROS), heat shock protein 60 (HSP 60), and upregulation of enzymes of the tricarboxylic acid cycle (TCA) and the respiratory chain (RCE)
In summary, data on improvement of function of autophagy, ubiquitination, modulation of micro RNA, and regulation of calcium homeostasis are available in trained skeletal and myocardial muscle cells, while nitric oxide metabolism is positively influenced through exercise in endothelial cells [6]. Evidence from a Wistar rat model suggests that exercise also activates myocardial stem cells [203], which may bear potential for exercise regimens in patients suffering from cancer treatment-related cardiac dysfunction. Although it could be hypothesized that exercise may exert a differential, pleiotropic effect on cancer and physiological cells to preserve CRF, antagonize tumor growth, and reduce side effects of cancer treatment, there is insufficient data on which cancer- induced impairments of intracellular pathways can be positively modulated through exercise. This can only be achieved through translational studies analyzing both clinical endpoints and molecular mechanisms to resolve the inherent threat that exercise may not only protect physiological but also cancer cells [70].
Cardiorespiratory fitness (CRF) and its assessment
The first documentation of a protective effect of swimming against cancer growth was demonstrated in a mouse model in 1952 [163]. While PA protected against cancer in humans and lowered cancer incidence by 48% and cancer-related mortality by 27% [198], cancer patients seem to be more sedative than adult controls and recommendations for PA are frequently (53–70%) not put into practice by cancer survivors [20]. Apparently healthy individuals and cardiooncological patients display a wide range of CRF, a predictor of cardiovascular morbidity and development of heart failure [94, 101, 145]. An improvement of 1 metabolic equivalent (MET, which is 3.5 ml/kg/min) of exercise performance has been associated with a 10–25% relative risk reduction in overall mortality [90], while low CRF has been associated with higher morbidity, poor quality of life, reduced cardiac performance during exercise and a worse cardiovascular risk profile in cancer patients [44]. In a single-centre cohort analysis of 1632 patients (58% male; 64 ± 12 years) with adult-onset cancer, treadmill testing was performed at a median of 7 years after primary cancer diagnosis. The adjusted risk of all-cause, cardiovascular, and cancer mortality decreased by 26%, 14%, and 25% with each one MET (3.5mlO2/kg/min) increment in CRF [61]. CRF decreases between 5 and 26% during exposure to different systemic treatment regimens [80, 84] and does not entirely recover following cessation of treatment [3, 85]. Currently, no reimbursement of structured exercise training as a part of the rehabilitation process of cancer patients has been established, despite its proven benefit on CRF improvement of cancer patients [103, 107].
CPET is the gold standard to assess CRF [62]. Evidence in a cancer population is mainly limited to pre-operative risk stratification, especially in lung [64], colon [213], and rectal [214] cancer. Whether CPET can predict cardiovascular events if it is performed prior to cardiotoxic cancer treatment is unknown. Current guidelines recommend CPET as to be considered in cancer patients with exertional limitations, especially those treated with higher doses of anthracyclines and/or radiotherapy including the heart [122]. CPET is considered useful if high cardiovascular toxicity is to be expected at baseline as well as in patients who develop chemotherapy-related cardiac disease during cancer treatment [122]. CPET is suggested if left ventricular function declines during or after therapy [57] and if subjective exercise limitations need to be differentiated into cardiac vs. non-cardiac origin, also in combination with stress echocardiography [183]. CPET could also be used to detect early deterioration of CRF in patients under immune-checkpoint inhibitors in addition to repetitive troponin measurements to better risk stratify cancer patients [201].
Using CPET a holistic and integrative approach could be taken in cancer patients to early diagnose and adequately train this vulnerable group [112, 157]. As many cancer patients display preserved ejection fraction, guidance could be retrieved from the position paper of exercise testing in heart failure with preserved ejection fraction [63]. Ejection fraction as such has been shown to be a modest marker to express CRF, and maximal as well as submaximal CPET variables may add benefits to guide therapy and follow-up visits [204, 207, 209, 212]. A depiction of useful CPET variables, most of which still have to be validated in a cancer cohort, is listed in Table 1.
Components of physical fitness and exercise
Sports cardiology recommendations propose a weekly “dose” of PA (which depicts unstructured activity as opposed to structured exercise training) of at least 150 min of moderate intensity, preferably on most days of the week [154]. This recommendation can be applied to cancer patients, since a meta-analysis on 50.000 breast and colon cancer patients has found a reduction of total mortality risk of 24% and 28% in active cancer survivors exercising for 150 min per week compared to sedentary controls [176]. Benefits of exercise training are not limited to improvement of cardiac function, but have beneficial effects on metabolic, muscular, motor, and morphological aspects of the human organism (see Fig. 4) [154]. Exercise training can also be performed in severely compromised patients if expertise in exercise sciences and training are available [206, 208].
Guidelines of sports cardiology also recommend variations in exercise frequency (sessions/week and bouts of exercise), intensity, duration (per day or week), or type (resistance, endurance, speed, flexibility, coordination) [154].
Endurance training recommendations are derived from % of VO2peak, % of peak heart rate, or % of heart rate reserve (the difference of resting and peak heart rate) [154]. Using % of VO2peak as a determinant to recommend endurance exercise prescription requires full metabolic exertion on initial CPET testing (at least requiring a respiratory exchange ratio, RER, > 1.05), which may be hard to achieve in severely compromised patients, such as those exercising during chemotherapy or advanced heart failure [41]. Comparison of VO2peak is limited between treadmill and bicycle testing because efficiency of oxygen consumption, among others, depends on applied muscle mass and body composition, with deviations of up to 20% [124, 150]. Current exercise physiology units and software specifications often apply the validated Wasserman-Hansen equation for reference values of VO2peak [66], but recent data suggest that adapted reference values gained from the FRIEND registry may be superior to anticipate morbidity [31, 144, 155].
Determination of exercise corridors from heart rate has limitations because individual heart rate performance curves or the application of beta- blockers complicate adequate prescriptions [74, 219]. An easy and practical approach to steer endurance exercise intensity is the BORG scale [21], which depicted physiological measures in a large young male and female Caucasian cohort (n = 2560, median age 28, IQR 17–44 years) [175]. Standard corridors for endurance training are shown in Table 2 [154]:
Intensity of RT is commonly determined with the one repetition maximum (1RPM). Moderate intensity between 30 and 50% of 1RPM with 15–30 repetitions is considered to be muscular endurance training [154]. In cardiac patients eight to ten resistance exercises are still recommended to cover most large muscular groups [154, 180] with resting intervals of 3-5 minutes [156]. In cancer patients there is limited data on comparisons between types of RT, such as dynamic vs. static/isometric exercise, which may create different muscular responses and differential effects on long-term blood pressure [154]. A meta-analysis of eleven randomized trials on cancer survivors exercising during and after chemotherapy showed that upper limb strength seems to improve slightly better at low-to-moderate intensities below 75% of 1RPM (p = 0.042) [186].
Clinical effects of exercise training in cancer patients
The effects of exercise on cardiovascular disease, heart failure, mortality, and surrogate variables have primarily been studied in breast cancer patients (see Appendix). The Framingham risk score (FRS) was higher in overweight early- stage breast cancer patients [15, 58]. Following AET and RT, FRS was significantly reduced in the intervention group corresponding to an 11% (CI: − 15 to − 5%) reduction on the FRS-predicted 10-year risk of developing cardiovascular disease [110].
The association between PA and cardiovascular disease was investigated in a prospective study with non-metastasized breast cancer patients (n = 2973, mean age 57 years, median follow-up of 8.6 years): Exercise was measured by MET-h/week and patients were classified into three groups: (1) 2–10.9, 11–24.5, and 24.5 MET-h/week. Cardiovascular events decreased across increasing total MET-h/wk categories (p < 0.001) in multivariable analysis. Compared with < 2 MET-h/week, the adjusted HR was 0.91 (CI: 0.76 to 1.09) for 2 to 10.9 MET-h/week, 0.79 (CI: 0.66 to 0.96) for 11 to 24.5 MET-h/week, and 0.65 (CI: 0.53 to 0.80) for ≥ 24.5 MET-h/week. A similar trend was observed for the incidence of coronary artery disease and heart failure (p values < 0.05). Importantly, further analysis revealed that adherence to national exercise guidelines for adult patients with cancer (≥ 9 MET-h/week) was associated with an adjusted 23% reduction in the risk of cardiovascular events in comparison to < 9 MET-h/week (p < 0.001; Appendix) [87]. Similarly, the large Women`s Health Initiative (WHI) study on non-metastatic breast cancer patients (n = 4015) with a long-term follow-up (median 12.7 years) also demonstrated a reduction of cardiovascular events with higher self-reported exercise levels prior or at the time of cancer diagnosis: Comparing ≥ 9MET-h/week (n = 1976) vs. < 9 MET-h/week (n = 2039), HR was 0.77 (CI: 0.62 to 0.95) for age-adjusted cardiovascular events and 0.56 (CI: 0.35 to 0.89) for coronary heart disease associated death [149, 151].
In breast cancer patients, trials demonstrated benefits of training in chemotherapy naïve patients [146], during neoadjuvant chemotherapy [86], during [37, 78] and after adjuvant chemotherapy [52, 67, 69, 87, 110], and during maintenance therapy on aromatase inhibitors [82] (see Appendix). Typical symptoms during radiotherapy, such as fatigue or arthralgia, were reduced through AET [118] and tumor relapse may be reduced in physically active individuals [7]. A meta-analysis of exercise in breast cancer patients included 22 prospective cohort studies (follow- up of 4.3 to 12.7 years, 123 574 participants, 6898 all-cause deaths, 5462 breast cancer-related deaths or recurrences) and showed fewer events for tumor progression (HR = 0.72, CI: 0.56–0.91) and tumor relapse (HR = 0.79, CI: 0.63–0.98) if women exercised before and after the diagnosis [105]. Patients reporting higher PA on tumor diagnosis demonstrated lower risk of all-cause (HR = 0.82, CI: 0.70–0.96) and breast cancer-related death (HR = 0.73, CI: 0.54–0.98) than more sedentary controls. However, this large meta-analysis also highlighted the heterogeneity of studies and variation of defining and assessing PA and AET [105].
Outcome data on exercise trials in urogenital cancer is scarce, with exercise trials showing a positive effect on CRF [5] and adverse health outcomes [53] in testicular cancer survivors. Similarly, CRF was improved [36] and tumor progression was reduced [165] in exercising prostate cancer patients (for details see Appendix).
Data on cardiovascular outcome or overall mortality reduction in colon cancer exercise trials are not yet available, but AET reduced post-operative complications [197], improved disease-free survival [131], quality of life [22], and decreased circulating markers associated with the formation of micro-metastasis, such as soluable intercellular adhesion molecule-1 [152] (for details see Appendix).
Exercise trials in lung cancer patients following surgery is scarce and no studies are available on benefits in overall survival due to exercise interventions. However, a meta-analysis of lung cancer exercise trials demonstrated that pre-operative exercise-based training can improve vital capacity and forced expiratory volume (standardized mean difference: 0.38 l, CI: 0.14–0.63 l and 0.27 l, CI: 0.11–0.42 l) before surgery and reduces in-hospital length of stay (mean difference in hospital days: − 4.83 days, CI: − 5.9–3.76 days) as well as post-operative complications (risk ratio: 0.45, CI: 0.28–0.74) after lung resection surgery [179]. A Cochrane meta-analysis of randomized controlled trials of participants with non-small cell lung cancer analyzed patients who had undergone lung resection [29]: Patients were allocated to receive either AET, RT, or a combination of both. Compared to usual care (UC), VO2peak (mean difference 2.97 ml/kg/min, CI: 1.93–4.02 ml/kg/min) and 6-min walk distance (6MWD, mean difference: 57 m, CI 34–80 m) were greater in the intervention group. In addition, improved force-generating capacity of the quadriceps muscle (mean difference 0.75, CI 0.4–1.1) was displayed [29].
Data on exercise interventions in head and neck cancer (HNC) patients are scare [26], but reduced loss of muscle mass [170] and improvement of functional capacity has been reported in HNC patients [8, 168, 169] (see Appendix).
Randomized trials on exercise-based reduction of cardiovascular events or mortality are not available in hematological cancers. No improvement of 6MWD was achieved in an exercise trial in patients with acute leukemia [23], while exercise intervention was superior to UC in surrogate markers such as quality of life and CRF in patients with Hodgkin and non-Hodgkin lymphoma [38] (see Appendix).
Limitations, recommendations and future directions- a critical appraisal
Current guidelines on cardiooncology acknowledge the role of cardiac rehabilitation and AET as a potent multi-targeted therapy to prevent and treat competing mechanisms of chemotherapy-related cardiovascular toxicity (CTR-CVT) [122]. Exercise is recommended to improve CRF in cancer patients [178], and to reduce the burden of cardiovascular injury and traditional cardiovascular risk factors [177]. To ensure optimized response to exercise, adaptation of basic principles of exercise sciences to improve CRF should be transferred to cancer patients [173]. In cancer patients several studies were performed in the form of home-based training, although the benefits of supervised training have been demonstrated [200]. CPET-based supervised training may best account for the individual exercise needs of this vulnerable group of patients.
CPET may not only be performed at baseline, but also could be repeated throughout the treatment process to re-evaluate CRF to detect early circulatory limitations, for instance decline or plateauing of O2 pulse as a surrogate for reduced stroke volume and peripheral oxygen extraction [210]. This may even necessitate the application of different CPET reference values for cancer patients [132]. Reduced CRF should not be merely separated into cardiac and non-cardiac origin, but one also needs to pay attention to reduced oxygen extraction, which may be a major limiting factor in cancer patients despite preserved ejection fraction [104]. It has been shown that in elderly patients with heart failure and preserved ejection fraction, VO2peak was better associated with arteriovenous oxygen extraction than cardiac output, highlighting the importance to analyze peripheral muscular response to exercise [68]; a phenomenon which can also be observed in cancer patients. Chemotherapy- induced loss of hemoglobin needs to be compensated by enhanced peripheral small-muscle blood flow and O2 extraction, which, however, is insufficient to maintain whole body performance [99]. Increased myosteatosis during chemotherapy suggests hampered fat oxidation [99]. In clinical practice, focusing on early (even before initiation of chemotherapy) aerobic-alactacid training may antagonize this process.
As VO2peak is dependent on metabolic exertion, non-exertional CPET variables, such as oxygen uptake efficiency slope [14], minute ventilation to carbon dioxide production, exercise oscillatory ventilation, and the cardiorespiratory optimal point may be of additional benefit in cancer patients, as this might prevent potential adverse events at peak exertion. Transition from aerobic to anaerobic metabolism can be illustrated by non-exertional variables, which may also be useful to monitor metabolic changes during cancer treatment. Combining right heart catheterization and CPET may complement the image to meticulously display exercise limitations and guide exercise training and drug therapy [205, 211].
Moderate continuous training (MCT), which is usually performed at 50–75% of peak heart rate, is still the backbone of AET. As opposed to MCT, HIIT uses repetitive bouts of high-intensity exercise intertwined by a variable duration of active recovery phases. The „Norwegian protocol “ originally used 4 x 4 min intervals at 90 and 95% of peak heart rate and included 3 min cool-down phases between 50 and 70% of peak heart rate using uphill treadmill walking [218]. HIIT improved VO2peak, endothelial and mitochondrial function as well as capillary density in the skeletal muscle [59, 123, 181]. A meta-analysis demonstrated higher efficacy to improve VO2peak in apparently healthy individuals [140], while a meta-analysis of heart failure trials showed a trend toward higher VO2peak improvement in HFrEF and even more so in HFpEF [59]. It has to be taken into account that vulnerable groups, such as HFrEF and cancer patients, may fail to sustain intensity corridors of the classical HIIT protocol [41], which may require adapted HIIT protocols with shorter bouts of high-intensity exercise and longer periods of recovery to avoid rapid lactate accumulation. Such adapted HIIT protocols are safe and effective in advanced heart failure patients with LVAD [9, 142, 208] and may prove useful in cancer patients.
In cancer patients supervised AET (including MCT and HIIT) is safe, well tolerated, and capable of improving VO2peak as well as attenuating CTR-CVT risk [202] and cardiovascular risk factors [110, 184]. A recent meta-analysis of controlled trials in cancer patients and survivors using post-intervention CPET did not find differences of VO2peak in MCT and HIIT (mean difference = 2.03 ml/kg/min, CI − 0.75–4.83 ml/kg/min) [202]. This conveys a slightly different effect compared to heart failure trials, which have shown a trend to more effective HIIT [59, 181]. This might be explained by the heterogeneity of exercise studies in cancer patients. We believe that it is essential to conduct studies using adapted HIIT in different cancer entities and states of disease (pre-, during-, and post-treatment) to increase the portfolio of available exercise programs. The need to establish more tailored exercise programs in cancer patients has been acknowledged by American [57] and European [122] recommendations. It also has to be highlighted that not only improvement of CRF should be achieved but also attenuation of chemotherapeutic side-effects, such as reduced quality of life and fatigue; HIIT is able to improve and maintain these effects for months following exercise intervention [4, 139]. Whether HIIT is also feasible in frail and older cancer patients still needs to be shown [126].
Performing AET during radiochemotherapy may need to be designed in a nonlinear periodization model to account for fluctuations of symptoms through therapeutic cycles without compromising the benefit of training [50, 52]. The Brexit trial is the first and only randomized controlled trial with a sufficient follow-up period to assess prevalence of functional disability (defined as VO2peak ≤ 18 ml/kg/min) after 1 year in an exercise training group compared to UC in breast cancer patients within two weeks of induction of chemotherapy (n = 52 per group, mean age 50.3 vs. 51.2 years). It made use of a combination of AET (MCT and HIIT) and RT. Importantly, both intensity and duration were progressively increased during macrocycles of exercise and were reduced by 5% in the week following anthracycline-based chemotherapy. Intensity of AET was based on % of heart rate reserve at the first ventilatory threshold (VT1) up to 85–95% of peak heart rate [52]. As no beta-blockers were prescribed in both groups, this approach to guide training intensity with heart rate monitoring (wrist watch) can be considered accurate. The Brexit trial used a supervised training protocol (three times/week for 30–60 min) for the first 12 weeks. AET was performed as MCT (20–30 beats/minute below %HRR at VT1) and HIIT, the later consisting of 4 x 2-4 minutes of interval training of % HRR at VT1 up to 95% of peak heart rate. It may be interesting to compare the efficacy of this approach with shorter bouts of HIIT. Importantly, the Brexit trial also implemented RT of various intensities between 60 and 70% (weeks 1–6) and 70–85% of 1RPM (following week 7) by using eight guided exercises of upper and lower muscle groups. It may be reasonable to modify these exercises with multi-component movements to recruit adjacent muscle chains and further improve intermuscular coordination. To study the response of the cardiovascular system around chemotherapy in more detail, it may be useful to perform CPET follow-up and lactate measurements during this critical period to delineate exercise limitations of cardiac output and peripheral oxygen extraction. This can be analyzed by CPET variables, such as flattening of peak O2pulse and VO2/W (Table 1), which have already shown prognostic [159] and pathophysiological impetus [210] in heart failure cohorts. Such variables should be analyzed and validated in cancer patients to survey response to chemotherapy. In addition, compound variables such as ventilatory [49] and circulatory power [34] could have the potential to better display CRF than VO2peak alone.
Limitations of exercise trials in sports cardio-oncology
Exercise trials are heterogeneous (see Appendix) because many aspects must be considered and sport scientific expertise is necessary to address the metabolic needs of vulnerable populations, such as cancer and/or heart failure patients: Steering RT intensities based on 1 RPM is reasonable to objectively measure intensity. However, in vulnerable populations, such as patients with cancer, advanced heart failure or left ventricular assist devices (LVAD) as well as exercise-naive patients, performing 1 RPM may not always be feasible. These patients often do not have the coordinative capabilities to perform such tests. In such cases a stepwise increase of RT intensity during the training cycle could be applied, with the main focus on building up smooth coordination of kinetic muscle chains. Our group has acknowledged this approach in an exercise training study in LVAD patients [208], which could also be adapted in a cancer population.
In the literature, no clear recommendations are provided for a major determinant of exercise limitation: Exercise density, which is the relationship between intensity and duration and the intertwined active or passive recovery periods. From a physiological point of view this aspect is of utmost importance when higher intensities are applied and local lactate clearance is a limiting factor, such as in heart failure and/or cancer patients. Studies with different exercise protocols on HIIT have been elaborated paying tribute to this methodological issue [9, 123, 208]. A large multi-center randomized trial in advanced heart failure patients showed that almost half of the patients did not adhere to the scheduled training corridors of HIIT [41], which may be due to limited availability of lactate measurements during training and may have been caused by early lactate accumulation and associated premature reduction of training intensity.
No uniform consensus exists on the determination of training corridors, while usually % of peak heart rate or heart rate reserve are used to separate MCT from HIIT. However, the heart rate performance curve varies considerably among individuals [74] and can be influenced by beta-blockers [219], which are often used in cancer patients with cardiovascular comorbidities; percentages of VO2peak or peak workload may be preferrable substitutes to guide training [208, 219]. It is noteworthy that great care should be taken in the interpretation of exercise studies, because terminology of prescribing exercise intensities based on ventilatory and lactate thresholds varies [19] (see Appendix). Comparability between MCT and HIIT is also hampered by the lack of isocaloric exercise stimuli, which is a prerequisite if effects on CRF are analyzed [59, 181]. Further studies comparing MCT and HIIT in cancer need to take this into account.
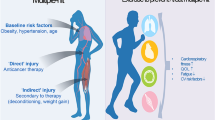
Data from randomized, controlled, supervised exercise interventions in cancer patients are available primarily in younger, non-cachectic individuals and do not include frail and sarcopenic patients [83, 130]. Sarcopenia and cachexia are a prominent complication in cancer patients and further promote CRF reduction (Fig. 5) [16]. Early recognition of sarcopenia and compilation of nutritional and exercise pathways are important goals in treating cancer patients [12, 46] and need to be addressed in future trials of sport cardio-oncology.
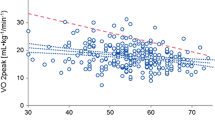
Exercise limitations of cancer patients. Cancer patients display reduced cardiorespiratory fitness (CRF) expressed through reduced peak oxygen consumption (VO2peak), which is often facilitated by cachexia and/or cancer-induced immobility. VO2peak reduction in cancer patients is a composite of lower cardiac output, stroke volume, heart rate increase, and peripheral oxygen extraction during exercise. Reduced left ventricular (LV) compliance and increased systemic vascular resistance also contribute to VO2peak reduction. Created with BioRender.com
Recommendations for exercise prescription in cancer patients
We believe that there cannot be a specific exercise program for each cancer entity, since exercise responses are expected to be different in each individual, also depending on (heart failure) co-medication, which may become necessary in the presence of cancer treatment-related cardiac dysfunction. As drug therapy may change before, during, and after chemotherapy we suggest repetitive CPET to achieve adequate metabolic corridors to sustain or even improve CRF. As there are many caveats of deriving exercise corridors from exertional variables, such as VO2peak [124] and peak heart rate [74], we suggest determination of AET exercise corridors from VT1 and the second ventilatory threshold (VT2). It should be noted that reduced VO2peak in cancer patients is the result of several mechanisms, including reduced cardiac output [51], reduced stroke volume increase [17], reduced left ventricular compliance [17, 83], lower heart rate response, higher systemic vascular resistance [18], impaired peripheral oxygen extraction [138], cachexia and immobility [10, 130]. Drug-related symptoms, such as fatigue or polyneuropathy, may also reduce adherence to exercise training and may ultimately reduce VO2peak during chemotherapy [96, 98]. An elegant study addressed this issue and performed 30 min of vigorous treadmill walking 24 h prior to every anthracycline therapy [97, 100], no adverse events and 100% adherence to exercise were achieved. Adherence to AET and symptoms refraining patients from participation in exercise sessions always need to be taken into account whenever effects of exercise interventions on VO2peak and other CPET variables are interpreted in cancer patients. A summary of cancer-induced exercise limitations is provided in Fig. 5:
HRR, the difference between resting and peak heart rate, which is often used in exercise trials, also has limitations: (1) Application of beta-blockers impacts on HRR, and (2) Taking resting heart rate prior to exercise testing may not represent “true” resting heart rate due to enhanced sympathetic tone in anticipation of testing. Thus, “true” resting heart rate may need to be recorded repetitively directly after waking up. This, however, is not a standard in trials of sports cardio-oncology. Implementation of a home-based monitoring in the form of wearables or apps should help to better assess “true” resting heart rate and have proven their benefit to raise awareness for physical activity in breast cancer patients [33, 42, 127]. An “app on prescription” is a promising new approach to increase the patient’s compliance to exercise and also aids to detect deterioration of health status and could be used in cancer similar to heart failure patients [1]. A combination of wearables and artificial intelligence-based approaches can be regarded promising to increase cancer detection [40, 108], treatment [221], as well as an improvement of lifestyle interventions [30] and early recognition of cardiac disease and heart failure [13, 56].
We suggest establishing exercise corridors above VT1: Moving beyond VT1, which is the first significant increase of ventilatory effort, can be considered the minimal stimulus to accomplish a training effect and should also be achieved in frail cancer patients. HIIT should be applied by short bouts close to VT2. Adaptations of intensity should be made during chemotherapy and CPET should be performed before, during, and after chemotherapy, as well as following adaptations of heart failure therapy. We also suggest combining MCT and HIIT, since both are safe and improve CRF.
An example of a tailored exercise periodization model for a breast cancer patient during and after chemotherapy is shown in Fig. 6:
An example for a tailored exercise prescription in a breast cancer patient undergoing 12 weeks of chemotherapy and a 12-month- follow-up is provided. Exercise is performed 3x/week (30 min each) including aerobic exercise training (AET) consisting of both moderate continuous (MCT) and high-intensity exercise training (HIIT) and additional resistance training (RT). A year of training is separated into four cycles of 12 weeks each, with the first cycle integrating chemotherapy. Before each cycle cardiopulmonary exercise testing (CPET) is performed to adapt training corridors of AET. MCT is performed at 5–10 beats/min (HR, heart rate) above the first ventilatory threshold (VT1), while HIIT consists of five intense cycles with 2 min each using short bouts just below (5–10 beats/minute) the second ventilatory threshold (VT2). RT is increased in intensity over the year starting with more repetitions (rep) and lower intensities (derived from the one repetition maximum, 1 RPM). Frequency of MCT and HIIT is varied across the cycles, RT is performed 3x/week. Created with BioRender.com
HIIT and MCT have beneficial effects on mitochondrial function and cell–cell communication, which both improve cellular function [24, 123]. In turn, these effects as efforts to protect the heart and cardiovascular system could possibly also protect cancer cells and remain a matter of concern [70,71,72]. Consequently, the differential effect of AET on preservation of CRF and simultaneously antagonizing cancer growth, metastasis, and metabolism needs to be further demonstrated by translational trials. These trials in sports cardio-oncology should incorporate clinical outcome measures, underlying microscopic mechanisms, and circulating biomarkers which may help in earlier detection of cancer therapeutics-related cardiac dysfunction [189].
Cardiovascular exercise response in cancer patients has primarily been analyzed with stress echocardiography and magnetic resonance imaging [83], although the gold standard to assess hemodynamics is right heart catheterization. There is a strong need to validate current results derived from imaging with simultaneous CPET and exercise right heart catheterization.
In summary, there is a strong need to create more homogeneity in cancer exercise trials, which can only be achieved by objective measurement of baseline performance, and more precise training surveillance [27, 125, 128]. This task can only be achieved through close interaction of physicians and exercise physiologists.
Conclusion
Aerobic exercise training is recommended as a part of adjuvant and neoadjuvant therapy as well as during chemotherapy [177, 220]. Unfortunately, randomized controlled trials using CPET prior to exercise initiation to adequately steer training intensity and improve cardiovascular and overall outcome are scarce in the cancer population. There is insufficient evidence on the most suitable type (supervised vs. home-based), content (endurance, resistance, or combined), intensity (moderate continuous vs. high-intensity interval), or density (short vs. longer bouts of stimuli with active vs. passive recovery periods) of training in cancer patients. Conducting randomized controlled exercise trials in patients undergoing radiochemotherapy are demanding and may be subject to significant drop-out rates during longer exercise intervention periods. Valid clinical endpoints, such as cardiovascular and overall mortality, are hard to achieve in younger cancer populations, who require a long follow-up period. Implementation of wearable devices into home-based exercise training and offers of public health providers (e.g., cancer sports groups) may improve adherence to AET and RT in cancer survivors.
Data availability
There was no collection of original data in this review. All information regarding this review will made available by the corresponding author on reasonable request.
Change history
26 February 2024
The article note stated that the article is part of a topical collection. However, this is not the case, the article is part of a special issue. This bug has been fixed.
References
(2024) DiGA ProHerz. In:ProCarement. https://procarement.com/patientinnen/diga/ [accessed 14.01.2024].
Abdel-Qadir H, Thavendiranathan P, Austin PC, Lee DS, Amir E, Tu JV, Fung K, Anderson GM (2019) The risk of heart failure and other cardiovascular hospitalizations after early stage breast cancer: a matched cohort study. J Natl Cancer Inst 111:854–862. https://doi.org/10.1093/jnci/djy218
Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, Greenbaum N, Mauch P, Lipshultz SE (2004) Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol 22:3139–3148. https://doi.org/10.1200/jco.2004.09.109
Adams SC, DeLorey DS, Davenport MH, Fairey AS, North S, Courneya KS (2018) Effects of high-intensity interval training on fatigue and quality of life in testicular cancer survivors. Br J Cancer 118:1313–1321. https://doi.org/10.1038/s41416-018-0044-7
Adams SC, DeLorey DS, Davenport MH, Stickland MK, Fairey AS, North S, Szczotka A, Courneya KS (2017) Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: A phase 2 randomized controlled trial. Cancer 123:4057–4065. https://doi.org/10.1002/cncr.30859
Adams V, Reich B, Uhlemann M, Niebauer J (2017) Molecular effects of exercise training in patients with cardiovascular disease: focus on skeletal muscle, endothelium, and myocardium. Am J Physiol Heart Circ Physiol 313:H72-h88. https://doi.org/10.1152/ajpheart.00470.2016
Agostini D, Natalucci V, Baldelli G, De Santi M, Donati Zeppa S, Vallorani L, Annibalini G, Lucertini F, Federici A, Izzo R, Stocchi V, Barbieri E (2018) New insights into the role of exercise in inhibiting mTOR signaling in triple-negative breast cancer. Oxid Med Cell Longev 2018:5896786. https://doi.org/10.1155/2018/5896786
Allen SK, Brown V, White D, King D, Hunt J, Wainwright J, Emery A, Hodge E, Kehinde A, Prabhu P, Rockall TA, Preston SR, Sultan J (2022) Multimodal prehabilitation during neoadjuvant therapy prior to esophagogastric cancer resection: effect on cardiopulmonary exercise test performance, muscle mass and quality of Life-A pilot randomized clinical trial. Ann Surg Oncol 29:1839–1850. https://doi.org/10.1245/s10434-021-11002-0
Alvarez Villela M, Chinnadurai T, Salkey K, Furlani A, Yanamandala M, Vukelic S, Sims DB, Shin JJ, Saeed O, Jorde UP, Patel SR (2021) Feasibility of high-intensity interval training in patients with left ventricular assist devices: a pilot study. ESC Heart Fail 8:498–507. https://doi.org/10.1002/ehf2.13106
Ameri P, Canepa M, Anker MS, Belenkov Y, Bergler-Klein J, Cohen-Solal A, Farmakis D, López-Fernández T, Lainscak M, Pudil R, Ruschitska F, Seferovic P, Filippatos G, Coats A, Suter T, Von Haehling S, Ciardiello F, de Boer RA, Lyon AR, Tocchetti CG (2018) Cancer diagnosis in patients with heart failure: epidemiology, clinical implications and gaps in knowledge. Eur J Heart Fail 20:879–887. https://doi.org/10.1002/ejhf.1165
Anker MS, Lena A, Roeland EJ, Porthun J, Schmitz S, Hadzibegovic S, Sikorski P, Wilkenshoff U, Fröhlich AK, Ramer LV, Rose M, Eucker J, Rassaf T, Totzeck M, Lehmann LH, von Haehling S, Coats AJS, Friede T, Butler J, Anker SD, Riess H, Landmesser U, Bullinger L, Keller U, Ahn J (2023) Patient-reported ability to walk 4 m and to wash: New clinical endpoints and predictors of survival in patients with pre-terminal cancer. J Cachexia Sarcopenia Muscle 14:1670–1681. https://doi.org/10.1002/jcsm.13247
Arends J, Muscaritoli M, Anker S, Audisio R, Barazzoni R, Bosnjak S, Bossi P, Bowman J, Gijssels S, Krznarić Ž, Strasser F, Aapro M (2023) Overcoming barriers to timely recognition and treatment of cancer cachexia: sharing progress in cancer care task force position paper and call to action. Crit Rev Oncol Hematol 185:103965. https://doi.org/10.1016/j.critrevonc.2023.103965
Attia ZI, Harmon DM, Behr ER, Friedman PA (2021) Application of artificial intelligence to the electrocardiogram. Eur Heart J 42:4717–4730. https://doi.org/10.1093/eurheartj/ehab649
Baba R, Nagashima M, Goto M, Nagano Y, Yokota M, Tauchi N, Nishibata K (1996) Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol 28:1567–1572. https://doi.org/10.1016/s0735-1097(96)00412-3
Barroso LC, Muro EC, Herrera ND, Ochoa GF, Hueros JI, Buitrago F (2010) Performance of the Framingham and SCORE cardiovascular risk prediction functions in a non-diabetic population of a Spanish health care centre: a validation study. Scand J Prim Health Care 28:242–248. https://doi.org/10.3109/02813432.2010.518407
Bauer J, Morley JE, Schols A, Ferrucci L, Cruz-Jentoft AJ, Dent E, Baracos VE, Crawford JA, Doehner W, Heymsfield SB, Jatoi A, Kalantar-Zadeh K, Lainscak M, Landi F, Laviano A, Mancuso M, Muscaritoli M, Prado CM, Strasser F, von Haehling S, Coats AJS, Anker SD (2019) Sarcopenia: a time for action. an SCWD position paper. J Cachexia Sarcopenia Muscle 10:956–961. https://doi.org/10.1002/jcsm.12483
Beaudry RI, Haykowsky MJ, MacNamara JP, Tucker WJ, Rao R, Haley B, Sarma S (2022) Cardiac mechanisms for low aerobic power in anthracycline treated, older, long-term breast cancer survivors. Cardiooncology 8:8. https://doi.org/10.1186/s40959-022-00134-1
Beaudry RI, Howden EJ, Foulkes S, Bigaran A, Claus P, Haykowsky MJ, Gerche A (2019) Determinants of exercise intolerance in breast cancer patients prior to anthracycline chemotherapy. Physiol Rep 7:e13971. https://doi.org/10.14814/phy2.13971
Binder RK, Wonisch M, Corra U, Cohen-Solal A, Vanhees L, Saner H, Schmid JP (2008) Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. Eur J Cardiovasc Prev Rehabil 15:726–734. https://doi.org/10.1097/HJR.0b013e328304fed4
Blanchard CM, Courneya KS, Stein K (2008) Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol 26:2198–2204. https://doi.org/10.1200/jco.2007.14.6217
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Brown JC, Troxel AB, Ky B, Damjanov N, Zemel BS, Rickels MR, Rhim AD, Rustgi AK, Courneya KS, Schmitz KH (2018) Dose-response effects of aerobic exercise among colon cancer survivors: a randomized phase II trial. Clin Colorectal Cancer 17:32–40. https://doi.org/10.1016/j.clcc.2017.06.001
Bryant AL, Deal AM, Battaglini CL, Phillips B, Pergolotti M, Coffman E, Foster MC, Wood WA, Bailey C, Hackney AC, Mayer DK, Muss HB, Reeve BB (2018) The effects of exercise on patient-reported outcomes and performance-based physical function in adults with acute leukemia undergoing induction therapy: exercise and quality of life in acute leukemia (EQUAL). Integr Cancer Ther 17:263–270. https://doi.org/10.1177/1534735417699881
Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ (2008) Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol 586:151–160. https://doi.org/10.1113/jphysiol.2007.142109
Burtscher J, Soltany A, Visavadiya NP, Burtscher M, Millet GP, Khoramipour K, Khamoui AV (2023) Mitochondrial stress and mitokines in aging. Aging Cell 22:e13770. https://doi.org/10.1111/acel.13770
Bye A, Sandmael JA, Stene GB, Thorsen L, Balstad TR, Solheim TS, Pripp AH, Oldervoll LM (2020) Exercise and nutrition interventions in patients with head and neck cancer during curative treatment: a systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu12113233
Campodonico J, Contini M, Alimento M, Mapelli M, Salvioni E, Mattavelli I, Bonomi A, Agostoni P (2023) Physiology of exercise and heart failure treatments: cardiopulmonary exercise testing as a tool for choosing the optimal therapeutic strategy. Eur J Prev Cardiol 30:ii54–ii62. https://doi.org/10.1093/eurjpc/zwad189
Cardaci S, Zheng L, MacKay G, van den Broek NJ, MacKenzie ED, Nixon C, Stevenson D, Tumanov S, Bulusu V, Kamphorst JJ, Vazquez A, Fleming S, Schiavi F, Kalna G, Blyth K, Strathdee D, Gottlieb E (2015) Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat Cell Biol 17:1317–1326. https://doi.org/10.1038/ncb3233
Cavalheri V, Burtin C, Formico VR, Nonoyama ML, Jenkins S, Spruit MA, Hill K (2019) Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev 6:Cd009955. https://doi.org/10.1002/14651858.CD009955.pub3
Chiang PH, Wong M, Dey S (2021) Using wearables and machine learning to enable personalized lifestyle recommendations to improve blood pressure. IEEE J Transl Eng Health Med 9:2700513. https://doi.org/10.1109/jtehm.2021.3098173
Chiaranda G, Myers J, Arena R, Kaminsky L, Sassone B, Pasanisi G, Mandini S, Mazzoni G, Grazzi G (2021) Prognostic comparison of the FRIEND and Wasserman/Hansen peak VO2 equations applied to a submaximal walking test in outpatients with cardiovascular disease. Eur J Prev Cardiol 28:287–292. https://doi.org/10.1177/2047487319871728
Chicco AJ, Schneider CM, Hayward R (2006) Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. J Cardiovasc Pharmacol 47:182–189. https://doi.org/10.1097/01.fjc.0000199682.43448.2d
Chung IY, Jung M, Park YR, Cho D, Chung H, Min YH, Park HJ, Lee M, Lee SB, Chung S, Son BH, Ahn SH, Lee JW (2019) Exercise promotion and distress reduction using a mobile app-based community in breast cancer survivors. Front Oncol 9:1505. https://doi.org/10.3389/fonc.2019.01505
Cohen-Solal A, Tabet JY, Logeart D, Bourgoin P, Tokmakova M, Dahan M (2002) A non-invasively determined surrogate of cardiac power ('circulatory power’) at peak exercise is a powerful prognostic factor in chronic heart failure. Eur Heart J 23:806–814. https://doi.org/10.1053/euhj.2001.2966
Colbert LH, Westerlind KC, Perkins SN, Haines DC, Berrigan D, Donehower LA, Fuchs-Young R, Hursting SD (2009) Exercise effects on tumorigenesis in a p53-deficient mouse model of breast cancer. Med Sci Sports Exerc 41:1597–1605. https://doi.org/10.1249/MSS.0b013e31819f1f05
Cormie P, Galvão DA, Spry N, Joseph D, Chee R, Taaffe DR, Chambers SK, Newton RU (2015) Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int 115:256–266. https://doi.org/10.1111/bju.12646
Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Friedenreich CM, Yasui Y, Reid RD, Cook D, Jespersen D, Proulx C, Dolan LB, Forbes CC, Wooding E, Trinh L, Segal RJ (2013) Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst 105:1821–1832. https://doi.org/10.1093/jnci/djt297
Courneya KS, Sellar CM, Stevinson C, McNeely ML, Peddle CJ, Friedenreich CM, Tankel K, Basi S, Chua N, Mazurek A, Reiman T (2009) Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol 27:4605–4612. https://doi.org/10.1200/jco.2008.20.0634
D’Ascenzi F, Anselmi F, Fiorentini C, Mannucci R, Bonifazi M, Mondillo S (2021) The benefits of exercise in cancer patients and the criteria for exercise prescription in cardio-oncology. Eur J Prev Cardiol 28:725–735. https://doi.org/10.1177/2047487319874900
Dembrower K, Crippa A, Colón E, Eklund M, Strand F (2023) Artificial intelligence for breast cancer detection in screening mammography in Sweden: a prospective, population-based, paired-reader, non-inferiority study. Lancet Digit Health 5:e703–e711. https://doi.org/10.1016/s2589-7500(23)00153-x
Ellingsen Ø, Halle M, Conraads V, Støylen A, Dalen H, Delagardelle C, Larsen AI, Hole T, Mezzani A, Van Craenenbroeck EM, Videm V, Beckers P, Christle JW, Winzer E, Mangner N, Woitek F, Höllriegel R, Pressler A, Monk-Hansen T, Snoer M, Feiereisen P, Valborgland T, Kjekshus J, Hambrecht R, Gielen S, Karlsen T, Prescott E, Linke A (2017) High-intensity interval training in patients with heart failure with reduced ejection fraction. Circulation 135:839–849. https://doi.org/10.1161/circulationaha.116.022924
Ester M, Wagoner CW, Dreger J, Chen G, McDonough MH, McNeely ML, Culos-Reed SN (2023) Effectiveness of a self-monitoring app in supporting physical activity maintenance among rural canadians with cancer after an exercise oncology program: cluster randomized controlled trial. JMIR Cancer 9:e47187. https://doi.org/10.2196/47187
Fadol AP, Mouhayar E, Reyes-Gibby CC (2017) The use of cardiac resynchronization therapy in cancer patients with heart failure. J Clin Exp Res Cardiol. https://doi.org/10.15744/2394-6504.3.105
Fardman A, Banschick GD, Rabia R, Percik R, Fourey D, Segev S, Klempfner R, Grossman E, Maor E (2021) Cardiorespiratory fitness and survival following cancer diagnosis. Eur J Prev Cardiol 28:1242–1249. https://doi.org/10.1177/2047487320930873
Farrar GJ, Chadderton N, Kenna PF, Millington-Ward S (2013) Mitochondrial disorders: aetiologies, models systems, and candidate therapies. Trends Genet 29:488–497. https://doi.org/10.1016/j.tig.2013.05.005
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12:489–495. https://doi.org/10.1016/s1470-2045(10)70218-7
Finke D, Heckmann MB, Wilhelm S, Entenmann L, Hund H, Bougatf N, Katus HA, Frey N, Lehmann LH (2023) Coronary artery disease, left ventricular function and cardiac biomarkers determine all-cause mortality in cancer patients-a large monocenter cohort study. Clin Res Cardiol 112:203–214. https://doi.org/10.1007/s00392-022-02001-6
Fitzgerald MD, Tanaka H, Tran ZV, Seals DR (1997) Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol 83:160–165. https://doi.org/10.1152/jappl.1997.83.1.160
Forman DE, Guazzi M, Myers J, Chase P, Bensimhon D, Cahalin LP, Peberdy MA, Ashley E, West E, Daniels KM, Arena R (2012) Ventilatory power: a novel index that enhances prognostic assessment of patients with heart failure. Circ Heart Fail 5:621–626. https://doi.org/10.1161/circheartfailure.112.968529
Foulkes SJ, Howden EJ, Antill Y, Loi S, Salim A, Haykowsky MJ, Daly RM, Fraser SF, La Gerche A (2020) Exercise as a diagnostic and therapeutic tool for preventing cardiovascular morbidity in breast cancer patients- the BReast cancer EXercise InTervention (BREXIT) trial protocol. BMC Cancer 20:655. https://doi.org/10.1186/s12885-020-07123-6
Foulkes SJ, Howden EJ, Bigaran A, Janssens K, Antill Y, Loi S, Claus P, Haykowsky MJ, Daly RM, Fraser SF (2019) Persistent impairment in cardiopulmonary fitness after breast cancer chemotherapy. Med Sci Sports Exerc 51:1573–1581. https://doi.org/10.1249/mss.0000000000001970
Foulkes SJ, Howden EJ, Haykowsky MJ, Antill Y, Salim A, Nightingale SS, Loi S, Claus P, Janssens K, Mitchell AM, Wright L, Costello BT, Lindqvist A, Burnham L, Wallace I, Daly RM, Fraser SF, La Gerche A (2023) Exercise for the prevention of anthracycline-induced functional disability and cardiac dysfunction: the BREXIT study. Circulation 147:532–545. https://doi.org/10.1161/circulationaha.122.062814
Fung C, Sesso HD, Williams AM, Kerns SL, Monahan P, Abu Zaid M, Feldman DR, Hamilton RJ, Vaughn DJ, Beard CJ, Kollmannsberger CK, Cook R, Althouse S, Ardeshir-Rouhani-Fard S, Lipshultz SE, Einhorn LH, Fossa SD, Travis LB (2017) Multi-institutional assessment of adverse health outcomes among north american testicular cancer survivors after modern cisplatin-based chemotherapy. J Clin Oncol 35:1211–1222. https://doi.org/10.1200/jco.2016.70.3108
Gaglio D, Metallo CM, Gameiro PA, Hiller K, Danna LS, Balestrieri C, Alberghina L, Stephanopoulos G, Chiaradonna F (2011) Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol 7:523. https://doi.org/10.1038/msb.2011.56
Gaude E, Frezza C (2014) Defects in mitochondrial metabolism and cancer. Cancer Metab 2:10. https://doi.org/10.1186/2049-3002-2-10
Gautam N, Ghanta SN, Mueller J, Mansour M, Chen Z, Puente C, Ha YM, Tarun T, Dhar G, Sivakumar K, Zhang Y, Halimeh AA, Nakarmi U, Al-Kindi S, DeMazumder D, Al’Aref SJ (2022) Artificial intelligence wearables and remote monitoring for heart failure: current and future applications. Diagnostics (Basel). https://doi.org/10.3390/diagnostics12122964
Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, La Gerche A, Ligibel JA, Lopez G, Madan K, Oeffinger KC, Salamone J, Scott JM, Squires RW, Thomas RJ, Treat-Jacobson DJ, Wright JS (2019) Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from The American Heart Association. Circulation 139:e997–e1012. https://doi.org/10.1161/cir.0000000000000679
Goh LG, Welborn TA, Dhaliwal SS (2014) Independent external validation of cardiovascular disease mortality in women utilising Framingham and SCORE risk models: a mortality follow-up study. BMC Womens Health 14:118. https://doi.org/10.1186/1472-6874-14-118
Gomes Neto M, Durães AR, Conceição LSR, Saquetto MB, Ellingsen Ø, Carvalho VO (2018) High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: a systematic review and meta-analysis. Int J Cardiol 261:134–141. https://doi.org/10.1016/j.ijcard.2018.02.076
Gordon JW, Rungi AA, Inagaki H (1985) Hood DA (2001) Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J Appl Physiol 90:389–396. https://doi.org/10.1152/jappl.2001.90.1.389
Groarke JD, Payne DL, Claggett B, Mehra MR, Gong J, Caron J, Mahmood SS, Hainer J, Neilan TG, Partridge AH, Di Carli M, Jones LW, Nohria A (2020) Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes 6:315–322. https://doi.org/10.1093/ehjqcco/qcaa015
Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ (2018) 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J 39:1144–1161. https://doi.org/10.1093/eurheartj/ehw180
Guazzi M, Wilhelm M, Halle M, Van Craenenbroeck E, Kemps H, de Boer RA, Coats AJS, Lund L, Mancini D, Borlaug B, Filippatos G, Pieske B (2022) Exercise testing in heart failure with preserved ejection fraction: an appraisal through diagnosis, pathophysiology and therapy - A clinical consensus statement of the Heart Failure Association and European Association of Preventive Cardiology of the European Society of Cardiology. Eur J Heart Fail 24:1327–1345. https://doi.org/10.1002/ejhf.2601
Ha D, Mazzone PJ, Ries AL, Malhotra A, Fuster M (2016) The utility of exercise testing in patients with lung cancer. J Thorac Oncol 11:1397–1410. https://doi.org/10.1016/j.jtho.2016.04.021
Hadzibegovic S, Porthun J, Lena A, Weinländer P, Lück LC, Potthoff SK, Rösnick L, Fröhlich AK, Ramer LV, Sonntag F, Wilkenshoff U, Ahn J, Keller U, Bullinger L, Mahabadi AA, Totzeck M, Rassaf T, von Haehling S, Coats AJS, Anker SD, Roeland EJ, Landmesser U, Anker MS (2023) Hand grip strength in patients with advanced cancer: a prospective study. J Cachexia Sarcopenia Muscle 14:1682–1694. https://doi.org/10.1002/jcsm.13248
Hansen JE, Sue DY, Wasserman K (1984) Predicted values for clinical exercise testing. Am Rev Respir Dis 129:S49-55. https://doi.org/10.1164/arrd.1984.129.2P2.S49
Hayes SCSM, Spence R, Pyke C, Saunders C, Bashford J, Eakin E (2017) Can exercise influence survival following breast cancer: results from a randomised, controlled trial. J Clin Oncol 35:S10067–S10067. https://doi.org/10.1200/JCO.2017.35.15_suppl.10067
Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW (2011) Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 58:265–274. https://doi.org/10.1016/j.jacc.2011.02.055
Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI (2009) Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res 15:4963–4967. https://doi.org/10.1158/1078-0432.Ccr-09-0628
Heusch G (2023) Cardioprotection in cardio-oncology: a case for concern? Cardiovasc Res 119:e144–e145. https://doi.org/10.1093/cvr/cvad111
Heusch G (2020) Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol 17:773–789. https://doi.org/10.1038/s41569-020-0403-y
Heusch G, Rassaf T (2021) Protection from cardiotoxicity of cancer chemotherapy: a novel target for remote ischaemic conditioning? Cardiovasc Res 117:985–986. https://doi.org/10.1093/cvr/cvaa199
Hinrichs L, Mrotzek SM, Mincu RI, Pohl J, Röll A, Michel L, Mahabadi AA, Al-Rashid F, Totzeck M, Rassaf T (2020) Troponins and natriuretic peptides in cardio-oncology patients-data from the ECoR registry. Front Pharmacol 11:740. https://doi.org/10.3389/fphar.2020.00740
Hofmann P, Von Duvillard SP, Seibert FJ, Pokan R, Wonisch M, Lemura LM, Schwaberger G (2001) %HRmax target heart rate is dependent on heart rate performance curve deflection. Med Sci Sports Exerc 33:1726–1731. https://doi.org/10.1097/00005768-200110000-00017
Holloszy JO (1967) Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242:2278–2282
Holloway GP (2017) Nutrition and training influences on the regulation of mitochondrial adenosine diphosphate sensitivity and bioenergetics. Sports Med 47:13–21. https://doi.org/10.1007/s40279-017-0693-3
Hood DA, Memme JM, Oliveira AN, Triolo M (2019) Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol 81:19–41. https://doi.org/10.1146/annurev-physiol-020518-114310
Howden EJ, Bigaran A, Beaudry R, Fraser S, Selig S, Foulkes S, Antill Y, Nightingale S, Loi S, Haykowsky MJ, La Gerche A (2019) Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol 26:305–315. https://doi.org/10.1177/2047487318811181
Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano-Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke-Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S (2022) 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 43:3618–3731. https://doi.org/10.1093/eurheartj/ehac237
Hurria A, Jones L, Muss HB (2016) Cancer treatment as an accelerated aging process: assessment, biomarkers, and interventions. Am Soc Clin Oncol Educ Book 35:e516-522. https://doi.org/10.1200/edbk_156160
Irrcher I, Ljubicic V, Hood DA (2009) Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol 296:C116-123. https://doi.org/10.1152/ajpcell.00267.2007
Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, Fiellin M, Capozza S, Rothbard M, Zhou Y, Harrigan M, Sanft T, Schmitz K, Neogi T, Hershman D, Ligibel J (2015) Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol 33:1104–1111. https://doi.org/10.1200/jco.2014.57.1547
Foulkes SJ, Haykowsky MJ, Li T, Wang J, Kennedy M, Kirkham AA, Thompson RB, Ian Paterson D, La Gerche A, Pituskin E (2023) Determinants of impaired peak oxygen uptake in breast cancer survivors. JACC CardioOncol. https://doi.org/10.1016/j.jaccao.2023.11.005
Jarden M, Hovgaard D, Boesen E, Quist M, Adamsen L (2007) Pilot study of a multimodal intervention: mixed-type exercise and psychoeducation in patients undergoing allogeneic stem cell transplantation. Bone Marrow Transplant 40:793–800. https://doi.org/10.1038/sj.bmt.1705807
Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE 2nd, Douglas PS, Haykowsky M (2012) Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 30:2530–2537. https://doi.org/10.1200/jco.2011.39.9014
Jones LW, Fels DR, West M, Allen JD, Broadwater G, Barry WT, Wilke LG, Masko E, Douglas PS, Dash RC, Povsic TJ, Peppercorn J, Marcom PK, Blackwell KL, Kimmick G, Turkington TG, Dewhirst MW (2013) Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res (Phila) 6:925–937. https://doi.org/10.1158/1940-6207.Capr-12-0416
Jones LW, Habel LA, Weltzien E, Castillo A, Gupta D, Kroenke CH, Kwan ML, Quesenberry CP Jr, Scott J, Sternfeld B, Yu A, Kushi LH, Caan BJ (2016) Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol 34:2743–2749. https://doi.org/10.1200/jco.2015.65.6603
Ju J, Nolan B, Cheh M, Bose M, Lin Y, Wagner GC, Yang CS (2008) Voluntary exercise inhibits intestinal tumorigenesis in Apc(Min/+) mice and azoxymethane/dextran sulfate sodium-treated mice. BMC Cancer 8:316. https://doi.org/10.1186/1471-2407-8-316
Kalkan A, Metze C, Iliadis C, Körber MI, Baldus S, Pfister R (2023) Prognostic impact of cancer history in patients undergoing transcatheter mitral valve repair. Clin Res Cardiol. https://doi.org/10.1007/s00392-023-02266-5
Kaminsky LA, Arena R, Beckie TM, Brubaker PH, Church TS, Forman DE, Franklin BA, Gulati M, Lavie CJ, Myers J, Patel MJ, Piña IL, Weintraub WS, Williams MA (2013) The importance of cardiorespiratory fitness in the United States: the need for a national registry: a policy statement from the American Heart Association. Circulation 127:652–662. https://doi.org/10.1161/CIR.0b013e31827ee100
Karsli-Uzunbas G, Guo JY, Price S, Teng X, Laddha SV, Khor S, Kalaany NY, Jacks T, Chan CS, Rabinowitz JD, White E (2014) Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov 4:914–927. https://doi.org/10.1158/2159-8290.Cd-14-0363
Kaszak I, Witkowska-Piłaszewicz O, Niewiadomska Z, Dworecka-Kaszak B, Ngosa Toka F, Jurka P (2020) Role of cadherins in cancer-a review. Int J Mol Sci. https://doi.org/10.3390/ijms21207624
Kersting D, Mavroeidi IA, Settelmeier S, Seifert R, Schuler M, Herrmann K, Rassaf T, Rischpler C (2023) Molecular imaging biomarkers in cardiooncology: a view on established technologies and future perspectives. J Nucl Med 64:29s–38s. https://doi.org/10.2967/jnumed.122.264868
Khan H, Kunutsor S, Rauramaa R, Savonen K, Kalogeropoulos AP, Georgiopoulou VV, Butler J, Laukkanen JA (2014) Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study. Eur J Heart Fail 16:180–188. https://doi.org/10.1111/ejhf.37
Kim Y, Triolo M, Erlich AT, Hood DA (2019) Regulation of autophagic and mitophagic flux during chronic contractile activity-induced muscle adaptations. Pflugers Arch 471:431–440. https://doi.org/10.1007/s00424-018-2225-x
Kirkham AA, Bonsignore A, Bland KA, McKenzie DC, Gelmon KA, CL VANP, Campbell KL, (2018) Exercise prescription and adherence for breast cancer: one size does not FITT All. Med Sci Sports Exerc 50:177–186. https://doi.org/10.1249/mss.0000000000001446
Kirkham AA, Eves ND, Shave RE, Bland KA, Bovard J, Gelmon KA, Virani SA, McKenzie DC, Stöhr EJ, Waburton DER, Campbell KL (2018) The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: a RCT. Breast Cancer Res Treat 167:719–729. https://doi.org/10.1007/s10549-017-4554-4
Kirkham AA, Paterson DI, Prado CM, Mackey JR, Courneya KS, Pituskin E, Thompson RB (2018) Rationale and design of the Caloric Restriction and Exercise protection from Anthracycline Toxic Effects (CREATE) study: a 3-arm parallel group phase II randomized controlled trial in early breast cancer. BMC Cancer 18:864. https://doi.org/10.1186/s12885-018-4778-7
Kirkham AA, Pituskin E, Mackey JR, Grenier JG, Ian Paterson D, Haykowsky MJ, Thompson RB (2022) Longitudinal changes in skeletal muscle metabolism, oxygen uptake, and myosteatosis during cardiotoxic treatment for early-stage breast cancer. Oncologist 27:e748–e754. https://doi.org/10.1093/oncolo/oyac092
Kirkham AA, Shave RE, Bland KA, Bovard JM, Eves ND, Gelmon KA, McKenzie DC, Virani SA, Stöhr EJ, Warburton DER, Campbell KL (2017) Protective effects of acute exercise prior to doxorubicin on cardiac function of breast cancer patients: a proof-of-concept RCT. Int J Cardiol 245:263–270. https://doi.org/10.1016/j.ijcard.2017.07.037
Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H (2009) Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301:2024–2035. https://doi.org/10.1001/jama.2009.681
Korste S, Settelmeier S, Michel L, Odersky A, Stock P, Reyes F, Haj-Yehia E, Anker MS, Grüneboom A, Hendgen-Cotta UB, Rassaf T, Totzeck M (2023) Anthracycline therapy modifies immune checkpoint signaling in the heart. Int J Mol Sci. https://doi.org/10.3390/ijms24076052
Kwan G, Balady GJ (2012) Cardiac rehabilitation 2012: advancing the field through emerging science. Circulation 125:e369-373. https://doi.org/10.1161/circulationaha.112.093310
La Gerche A, Howden EJ, Haykowsky MJ, Lewis GD, Levine BD, Kovacic JC (2022) Heart failure with preserved ejection fraction as an exercise deficiency syndrome: JACC focus seminar 2/4. J Am Coll Cardiol 80:1177–1191. https://doi.org/10.1016/j.jacc.2022.07.011
Lahart IM, Metsios GS, Nevill AM, Carmichael AR (2015) Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol 54:635–654. https://doi.org/10.3109/0284186x.2014.998275
Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, Fisher CC, Zhang M, Saucerman JJ, Goodyear LJ, Kundu M, Yan Z (2017) Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun 8:548. https://doi.org/10.1038/s41467-017-00520-9
Lakoski SG, Eves ND, Douglas PS, Jones LW (2012) Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol 9:288–296. https://doi.org/10.1038/nrclinonc.2012.27
Lång K, Josefsson V, Larsson AM, Larsson S, Högberg C, Sartor H, Hofvind S, Andersson I, Rosso A (2023) Artificial intelligence-supported screen reading versus standard double reading in the Mammography Screening with Artificial Intelligence trial (MASAI): a clinical safety analysis of a randomised, controlled, non-inferiority, single-blinded, screening accuracy study. Lancet Oncol 24:936–944. https://doi.org/10.1016/s1470-2045(23)00298-x
Lawler M, Davies L, Oberst S, Oliver K, Eggermont A, Schmutz A, La Vecchia C, Allemani C, Lievens Y, Naredi P, Cufer T, Aggarwal A, Aapro M, Apostolidis K, Baird AM, Cardoso F, Charalambous A, Coleman MP, Costa A, Crul M, Dégi CL, Di Nicolantonio F, Erdem S, Geanta M, Geissler J, Jassem J, Jagielska B, Jonsson B, Kelly D, Kelm O, Kolarova T, Kutluk T, Lewison G, Meunier F, Pelouchova J, Philip T, Price R, Rau B, Rubio IT, Selby P, Južnič Sotlar M, Spurrier-Bernard G, van Hoeve JC, Vrdoljak E, Westerhuis W, Wojciechowska U, Sullivan R (2023) European Groundshot-addressing Europe’s cancer research challenges: a Lancet Oncology Commission. Lancet Oncol 24:e11–e56. https://doi.org/10.1016/s1470-2045(22)00540-x
Lee K, Tripathy D, Demark-Wahnefried W, Courneya KS, Sami N, Bernstein L, Spicer D, Buchanan TA, Mortimer JE, Dieli-Conwright CM (2019) Effect of aerobic and resistance exercise intervention on cardiovascular disease risk in women with early-stage breast cancer: a randomized clinical trial. JAMA Oncol 5:710–714. https://doi.org/10.1001/jamaoncol.2019.0038
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS (2017) Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer 16:10. https://doi.org/10.1186/s12943-016-0577-4
Lehmann LH, Cautela J, Palaskas N, Baik AH, Meijers WC, Allenbach Y, Alexandre J, Rassaf T, Müller OJ, Aras M, Asnani AH, Deswal A, Laufer-Perl M, Thuny F, Kerneis M, Hayek SS, Ederhy S, Salem JE, Moslehi JJ (2021) Clinical strategy for the diagnosis and treatment of immune checkpoint inhibitor-associated myocarditis: a narrative review. JAMA Cardiol 6:1329–1337. https://doi.org/10.1001/jamacardio.2021.2241
Lehmann LH, Stein F, Jäger D, Frey N (2022) The Heidelberg cardio-oncology unit (COUNT)-a possible blueprint for improved care of cardio-oncological patients. Clin Res Cardiol 111:227–229. https://doi.org/10.1007/s00392-021-01894-z
Lena A, Wilkenshoff U, Hadzibegovic S, Porthun J, Rösnick L, Fröhlich AK, Zeller T, Karakas M, Keller U, Ahn J, Bullinger L, Riess H, Rosen SD, Lyon AR, Lüscher TF, Totzeck M, Rassaf T, Burkhoff D, Mehra MR, Bax JJ, Butler J, Edelmann F, Haverkamp W, Anker SD, Packer M, Coats AJS, von Haehling S, Landmesser U, Anker MS (2023) Clinical and prognostic relevance of cardiac wasting in patients with advanced cancer. J Am Coll Cardiol 81:1569–1586. https://doi.org/10.1016/j.jacc.2023.02.039
Leung PS, Aronson WJ, Ngo TH, Golding LA, Barnard RJ (2004) Exercise alters the IGF axis in vivo and increases protein in prostate tumor cells in vitro. J Appl Physiol 96:450–454. https://doi.org/10.1152/japplphysiol.00871.2003
Lind A, Totzeck M, Mahabadi AA, Jánosi RA, El Gabry M, Ruhparwar A, Mrotzek SM, Hinrichs L, Akdeniz M, Rassaf T, Mincu RI (2020) Impact of cancer in patients undergoing transcatheter aortic valve replacement: a single-center study. JACC CardioOncol 2:735–743. https://doi.org/10.1016/j.jaccao.2020.11.008
Linehan WM, Srinivasan R, Garcia JA (2013) Non-clear cell renal cancer: disease-based management and opportunities for targeted therapeutic approaches. Semin Oncol 40:511–520. https://doi.org/10.1053/j.seminoncol.2013.05.009
Lipsett A, Barrett S, Haruna F, Mustian K, O’Donovan A (2017) The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: a systematic review and meta-analysis. Breast 32:144–155. https://doi.org/10.1016/j.breast.2017.02.002
Liu Y, Sun Y, Guo Y, Shi X, Chen X, Feng W, Wu LL, Zhang J, Yu S, Wang Y, Shi Y (2023) An overview: the diversified role of mitochondria in cancer metabolism. Int J Biol Sci 19:897–915. https://doi.org/10.7150/ijbs.81609
Lonardo E, Cioffi M, Sancho P, Sanchez-Ripoll Y, Trabulo SM, Dorado J, Balic A, Hidalgo M, Heeschen C (2013) Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS ONE 8:e76518. https://doi.org/10.1371/journal.pone.0076518
Lundby C, Jacobs RA (2016) Adaptations of skeletal muscle mitochondria to exercise training. Exp Physiol 101:17–22. https://doi.org/10.1113/ep085319
Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH (2022) 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 43:4229–4361. https://doi.org/10.1093/eurheartj/ehac244
MacInnis MJ, Gibala MJ (2017) Physiological adaptations to interval training and the role of exercise intensity. J Physiol 595:2915–2930. https://doi.org/10.1113/jp273196
Malhotra R, Bakken K, D’Elia E, Lewis GD (2016) Cardiopulmonary exercise testing in heart failure. JACC Heart Fail 4:607–616. https://doi.org/10.1016/j.jchf.2016.03.022
Mapelli M, Salvioni E, Mattavelli I, Gugliandolo P, Bonomi A, Palermo P, Rossi M, Stolfo D, Gustafsson F, Piepoli M, Agostoni P (2023) Activities of daily living in heart failure patients and healthy subjects: when the cardiopulmonary assessment goes beyond traditional exercise test protocols. Eur J Prev Cardiol 30:ii47–ii53. https://doi.org/10.1093/eurjpc/zwad155
Marriott CFS, Petrella AFM, Marriott ECS, Boa Sorte Silva NC, Petrella RJ (2021) High-intensity interval training in older adults: a scoping review. Sports Med Open 7:49. https://doi.org/10.1186/s40798-021-00344-4
Martín-Payo R, Martínez-Urquijo A, Zabaleta-Del-Olmo E, Del Mar F-A (2023) Use a web-app to improve breast cancer risk factors and symptoms knowledge and adherence to healthy diet and physical activity in women without breast cancer diagnosis (Precam project). Cancer Causes Control 34:113–122. https://doi.org/10.1007/s10552-022-01647-x
Mattavelli I, Vignati C, Farina S, Apostolo A, Cattadori G, De Martino F, Pezzuto B, Zaffalon D, Agostoni P (2023) Beyond VO2: the complex cardiopulmonary exercise test. Eur J Prev Cardiol 30:ii34–ii39. https://doi.org/10.1093/eurjpc/zwad154
Mattioli R, Ilari A, Colotti B, Mosca L, Fazi F, Colotti G (2023) Doxorubicin and other anthracyclines in cancers: activity, chemoresistance and its overcoming. Mol Aspects Med 93:101205. https://doi.org/10.1016/j.mam.2023.101205
McDonald J, Sayers J, Anker SD, Arends J, Balstad TR, Baracos V, Brown L, Bye A, Dajani O, Dolan R, Fallon MT, Fraser E, Griel C, Grzyb A, Hjermstad M, Jamal-Hanjani M, Jakobsen G, Kaasa S, McMillan D, Maddocks M, Philips I, Ottestad IO, Reid KF, Sousa MS, Simpson MR, Vagnildhaug OM, Skipworth RJE, Solheim TS, Laird BJA (2023) Physical function endpoints in cancer cachexia clinical trials: Systematic Review 1 of the cachexia endpoints series. J Cachexia Sarcopenia Muscle 14:1932–1948. https://doi.org/10.1002/jcsm.13321
Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS (2006) Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 24:3535–3541. https://doi.org/10.1200/jco.2006.06.0863
Michalski M, Rowed K, Lavery JA, Moskowitz CS, Capaci C, Stene G, Edvardsen E, Eves ND, Jones LW, Scott JM (2022) Validity of estimated cardiorespiratory fitness in patients with primary breast cancer. JACC CardioOncol 4:210–219. https://doi.org/10.1016/j.jaccao.2022.05.003
Michel L, Helfrich I, Hendgen-Cotta UB, Mincu RI, Korste S, Mrotzek SM, Spomer A, Odersky A, Rischpler C, Herrmann K, Umutlu L, Coman C, Ahrends R, Sickmann A, Löffek S, Livingstone E, Ugurel S, Zimmer L, Gunzer M, Schadendorf D, Totzeck M, Rassaf T (2022) Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J 43:316–329. https://doi.org/10.1093/eurheartj/ehab430
Michel L, Mincu RI, Mahabadi AA, Settelmeier S, Al-Rashid F, Rassaf T, Totzeck M (2020) Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail 22:350–361. https://doi.org/10.1002/ejhf.1631
Michel L, Mincu RI, Mrotzek SM, Korste S, Neudorf U, Rassaf T, Totzeck M (2020) Cardiac biomarkers for the detection of cardiotoxicity in childhood cancer-a meta-analysis. ESC Heart Fail 7:423–433. https://doi.org/10.1002/ehf2.12589
Michel L, Rassaf T, Totzeck M (2018) Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J Thorac Dis 10:S4282-s4295. https://doi.org/10.21037/jtd.2018.08.15
Michel L, Rassaf T, Totzeck M (2019) Cardiotoxicity from immune checkpoint inhibitors. Int J Cardiol Heart Vasc 25:100420. https://doi.org/10.1016/j.ijcha.2019.100420
Mijwel S, Cardinale DA, Norrbom J, Chapman M, Ivarsson N, Wengström Y, Sundberg CJ, Rundqvist H (2018) Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. Faseb J 32:5495–5505. https://doi.org/10.1096/fj.201700968R
Mijwel S, Jervaeus A, Bolam KA, Norrbom J, Bergh J, Rundqvist H, Wengström Y (2019) High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J Cancer Surviv 13:244–256. https://doi.org/10.1007/s11764-019-00747-z
Milanović Z, Sporiš G, Weston M (2015) Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med 45:1469–1481. https://doi.org/10.1007/s40279-015-0365-0
Mincu RI, Mahabadi AA, Michel L, Mrotzek SM, Schadendorf D, Rassaf T, Totzeck M (2019) Cardiovascular adverse events associated with BRAF and MEK inhibitors: a systematic review and meta-analysis. JAMA Netw Open 2:e198890. https://doi.org/10.1001/jamanetworkopen.2019.8890
Moreno-Suarez I, Scheer A, Lam K, Dembo L, Spence AL, Hayward C, Kaye DM, Leet A, Fuller LM, Jacques A, Naylor LH, Green DJ, Maiorana A (2020) High-intensity interval training in patients with left ventricular assist devices: a pilot randomized controlled trial. J Heart Lung Transplant 39:1380–1388. https://doi.org/10.1016/j.healun.2020.08.005
Mrotzek SM, Lena A, Hadzibegovic S, Ludwig R, Al-Rashid F, Mahabadi AA, Mincu RI, Michel L, Johannsen L, Hinrichs L, Schuler M, Keller U, Anker SD, Landmesser U, Rassaf T, Anker MS, Totzeck M (2021) Assessment of coronary artery disease during hospitalization for cancer treatment. Clin Res Cardiol 110:200–210. https://doi.org/10.1007/s00392-020-01719-5
Myers J, Kaminsky LA, Lima R, Christle JW, Ashley E, Arena R (2017) A Reference equation for normal standards for VO(2) the fitness registry and the importance of exercise national database (FRIEND Registry). Prog Cardiovasc Dis 60:21–29. https://doi.org/10.1016/j.pcad.2017.03.002
Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE (2002) Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346:793–801. https://doi.org/10.1056/NEJMoa011858
Nagy AC, GulAcsi BP, CserEp Z, Hangody L, Forster T (2017) Late cardiac effect of anthracycline therapy in physically active breast cancer survivors - a prospective study. Neoplasma 64:92–100. https://doi.org/10.4149/neo_2017_111
Ohtani K, Fujino T, Ide T, Funakoshi K, Sakamoto I, Hiasa KI, Higo T, Kamezaki K, Akashi K, Tsutsui H (2019) Recovery from left ventricular dysfunction was associated with the early introduction of heart failure medical treatment in cancer patients with anthracycline-induced cardiotoxicity. Clin Res Cardiol 108:600–611. https://doi.org/10.1007/s00392-018-1386-0
Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO (2003) Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. Faseb J 17:675–681. https://doi.org/10.1096/fj.02-0951com
Okwuosa TM, Ray RM, Palomo A, Foraker RE, Johnson L, Paskett ED, Caan B, Jones LW (2019) Pre-Diagnosis exercise and cardiovascular events in primary breast cancer: women’s health initiative. JACC CardioOncol 1:41–50. https://doi.org/10.1016/j.jaccao.2019.08.014
Osman AF, Mehra MR, Lavie CJ, Nunez E, Milani RV (2000) The incremental prognostic importance of body fat adjusted peak oxygen consumption in chronic heart failure. J Am Coll Cardiol 36:2126–2131. https://doi.org/10.1016/s0735-1097(00)00985-2
Palomo ARR, Johnson L, Paskett E, Caan B, Jones L, Okwuosa T (2017) Associations between exercise prior to and around the time of cancer diagnosis and subsequent cardiovascular events in women with breast cancer: a women’s health initiative (WHI) analysis. J Am Coll Cardiol 69(11):1774
Paschos KA, Canovas D, Bird NC (2009) The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cell Signal 21:665–674. https://doi.org/10.1016/j.cellsig.2009.01.006
Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, Nielsen J, Gehl J, Pedersen BK, Thor Straten P, Hojman P (2016) Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab 23:554–562. https://doi.org/10.1016/j.cmet.2016.01.011
Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos-Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M (2021) 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 42:17–96. https://doi.org/10.1093/eurheartj/ehaa605
Peterman JE, Arena R, Myers J, Marzolini S, Ades PA, Savage PD, Lavie CJ, Kaminsky LA (2021) Reference standards for cardiorespiratory fitness by cardiovascular disease category and testing modality: data from FRIEND. J Am Heart Assoc 10:e022336. https://doi.org/10.1161/jaha.121.022336
Peterson MD, Rhea MR, Alvar BA (2005) Applications of the dose-response for muscular strength development: a review of meta-analytic efficacy and reliability for designing training prescription. J Strength Cond Res 19:950–958. https://doi.org/10.1519/r-16874.1
Pituskin E, Cox-Kennett N, Foulkes S, Driga A, Dimitry R, Thompson RB, Kirkham A, Prado C, Gyenes G, Haykowsky MJ (2023) Cardio-oncology and cancer rehabilitation: is an integrated approach possible? Can J Cardiol. https://doi.org/10.1016/j.cjca.2023.09.024
Pohl J, Mincu RI, Mrotzek SM, Wakili R, Mahabadi AA, Potthoff SK, Siveke JT, Keller U, Landmesser U, Rassaf T, Anker MS, Totzeck M (2021) ECG scoring for the evaluation of therapy-naïve cancer patients to predict cardiotoxicity. Cancers (Basel). https://doi.org/10.3390/cancers13061197
Popovic D, Arena R, Guazzi M (2018) A flattening oxygen consumption trajectory phenotypes disease severity and poor prognosis in patients with heart failure with reduced, mid-range, and preserved ejection fraction. Eur J Heart Fail 20:1115–1124. https://doi.org/10.1002/ejhf.1140
Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB (2015) Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 47:1922–1931. https://doi.org/10.1249/mss.0000000000000605
Puterman E, Lin J, Blackburn E, O’Donovan A, Adler N, Epel E (2010) The power of exercise: buffering the effect of chronic stress on telomere length. PLoS ONE 5:e10837. https://doi.org/10.1371/journal.pone.0010837
Ramin C, Schaeffer ML, Zheng Z, Connor AE, Hoffman-Bolton J, Lau B, Visvanathan K (2021) All-cause and cardiovascular disease mortality among breast cancer survivors in CLUE II, a long-standing community-based cohort. J Natl Cancer Inst 113:137–145. https://doi.org/10.1093/jnci/djaa096
Rashkis HA (1952) Systemic stress as an inhibitor of experimental tumors in Swiss mice. Science 116:169–171. https://doi.org/10.1126/science.116.3007.169
Rassaf T, Totzeck M, Backs J, Bokemeyer C, Hallek M, Hilfiker-Kleiner D, Hochhaus A, Lüftner D, Müller OJ, Neudorf U, Pfister R, von Haehling S, Lehmann LH, Bauersachs J (2020) Onco-cardiology: consensus paper of the german cardiac society, the german society for pediatric cardiology and congenital heart defects and the german society for hematology and medical oncology. Clin Res Cardiol 109:1197–1222. https://doi.org/10.1007/s00392-020-01636-7
Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM (2011) Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res 71:3889–3895. https://doi.org/10.1158/0008-5472.Can-10-3932
Rosenfeldt MT, O’Prey J, Morton JP, Nixon C, MacKay G, Mrowinska A, Au A, Rai TS, Zheng L, Ridgway R, Adams PD, Anderson KI, Gottlieb E, Sansom OJ, Ryan KM (2013) p53 status determines the role of autophagy in pancreatic tumour development. Nature 504:296–300. https://doi.org/10.1038/nature12865
Sale DG (1987) Influence of exercise and training on motor unit activation. Exerc Sport Sci Rev 15:95–151
Samuel SR, Maiya AG, Fernandes DJ, Guddattu V, Saxena PUP, Kurian JR, Lin PJ, Mustian KM (2019) Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Support Care Cancer 27:3913–3920. https://doi.org/10.1007/s00520-019-04750-z
Samuel SR, Maiya GA, Babu AS, Vidyasagar MS (2013) Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res 137:515–520
Sandmael JA, Bye A, Solheim TS, Stene GB, Thorsen L, Kaasa S, Lund J, Oldervoll LM (2017) Feasibility and preliminary effects of resistance training and nutritional supplements during versus after radiotherapy in patients with head and neck cancer: a pilot randomized trial. Cancer 123:4440–4448. https://doi.org/10.1002/cncr.30901
Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J, Ibrahim JG, Jolly TA, Williams G, Carey LA, Drobish A, Gordon BB, Alston S, Hurria A, Kleinhans K, Rudolph KL, Sharpless NE, Muss HB (2014) Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst 106:057. https://doi.org/10.1093/jnci/dju057
Santoro F, Tarantino N, Pellegrino PL, Caivano M, Lopizzo A, Di Biase M, Brunetti ND (2014) Cardiovascular sequelae of radiation therapy. Clin Res Cardiol 103:955–967. https://doi.org/10.1007/s00392-014-0718-y
Sasso JP, Eves ND, Christensen JF, Koelwyn GJ, Scott J, Jones LW (2015) A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle 6:115–124. https://doi.org/10.1002/jcsm.12042
Scarpulla RC, Vega RB, Kelly DP (2012) Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 23:459–466. https://doi.org/10.1016/j.tem.2012.06.006
Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M (2013) Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol 113:147–155. https://doi.org/10.1007/s00421-012-2421-x
Schmid D, Leitzmann MF (2014) Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol 25:1293–1311. https://doi.org/10.1093/annonc/mdu012
Scott JM, Nilsen TS, Gupta D, Jones LW (2018) Exercise therapy and cardiovascular toxicity in cancer. Circulation 137:1176–1191. https://doi.org/10.1161/circulationaha.117.024671
Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, Moskowitz CS, Matsoukas K, Iyengar NM, Dang CT, Jones LW (2018) Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol 36:2297–2305. https://doi.org/10.1200/jco.2017.77.5809
Sebio Garcia R, Yáñez Brage MI, Giménez Moolhuyzen E, Granger CL, Denehy L (2016) Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg 23:486–497. https://doi.org/10.1093/icvts/ivw152
Shephard RJ, Balady GJ (1999) Exercise as cardiovascular therapy. Circulation 99:963–972. https://doi.org/10.1161/01.cir.99.7.963
Siddiqi TJ, Rashid AM, Javaid SS, Siddiqi AK, Usman MS, Hervir O, Kamimura D, Lavie CJ, Mentz RJ, Butler J, Hall ME (2023) High-intensity interval training versus moderate continuous training in patients with heart failure with preserved ejection fraction: a systematic review and meta-analysis. Curr Probl Cardiol 48:101720. https://doi.org/10.1016/j.cpcardiol.2023.101720
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72:7–33. https://doi.org/10.3322/caac.21708
Smarż K, Jaxa-Chamiec T, Chwyczko T, Główczyńska R, Jegier A, Niedoszytko P, Piotrowicz E, Rybicki J, Straburzyńska-Migaj E, Szalewska D, Szmit S, Wolszakiewicz J (2019) Cardiopulmonary exercise testing in adult cardiology: expert opinion of the working group of cardiac rehabilitation and exercise physiology of the polish cardiac society. Kardiol Pol 77:730–756. https://doi.org/10.33963/kp.14889
Squires RW, Shultz AM, Herrmann J (2018) Exercise training and cardiovascular health in cancer patients. Curr Oncol Rep 20:27. https://doi.org/10.1007/s11912-018-0681-2
Stewart JB, Alaei-Mahabadi B, Sabarinathan R, Samuelsson T, Gorodkin J, Gustafsson CM, Larsson E (2015) Simultaneous DNA and RNA mapping of somatic mitochondrial mutations across diverse human cancers. PLoS Genet 11:e1005333. https://doi.org/10.1371/journal.pgen.1005333
Strasser B, Steindorf K, Wiskemann J, Ulrich CM (2013) Impact of resistance training in cancer survivors: a meta-analysis. Med Sci Sports Exerc 45:2080–2090. https://doi.org/10.1249/MSS.0b013e31829a3b63
Tabata N, Weber M, Sugiura A, Öztürk C, Tsujita K, Nickenig G, Sinning JM (2021) Impact of cancer history on clinical outcome in patients undergoing transcatheter edge-to-edge mitral repair. Clin Res Cardiol 110:440–450. https://doi.org/10.1007/s00392-020-01770-2
Tilemann LM, Heckmann MB, Katus HA, Lehmann LH, Müller OJ (2018) Cardio-oncology: conflicting priorities of anticancer treatment and cardiovascular outcome. Clin Res Cardiol 107:271–280. https://doi.org/10.1007/s00392-018-1202-x
Tonry C, Russell-Hallinan A, McCune C, Collier P, Harbinson M, Dixon L, Watson CJ (2023) Circulating biomarkers for management of cancer therapeutics-related cardiac dysfunction. Cardiovasc Res 119:710–728. https://doi.org/10.1093/cvr/cvac087
Totzeck M, Aide N, Bauersachs J, Bucerius J, Georgoulias P, Herrmann K, Hyafil F, Kunikowska J, Lubberink M, Nappi C, Rassaf T, Saraste A, Sciagra R, Slart R, Verberne H, Rischpler C (2023) Nuclear medicine in the assessment and prevention of cancer therapy-related cardiotoxicity: prospects and proposal of use by the European Association of Nuclear Medicine (EANM). Eur J Nucl Med Mol Imaging 50:792–812. https://doi.org/10.1007/s00259-022-05991-7
Totzeck M, Anker MS, Rassaf T (2023) CAR T-cell cancer therapies: do not forget the heart. Eur Heart J 44:2043–2045. https://doi.org/10.1093/eurheartj/ehad175
Totzeck M, Michel L, Lin Y, Herrmann J, Rassaf T (2022) Cardiotoxicity from chimeric antigen receptor-T cell therapy for advanced malignancies. Eur Heart J 43:1928–1940. https://doi.org/10.1093/eurheartj/ehac106
Totzeck M, Mincu RI, Heusch G, Rassaf T (2019) Heart failure from cancer therapy: can we prevent it? ESC Heart Fail 6:856–862. https://doi.org/10.1002/ehf2.12493
Totzeck M, Mincu RI, Mrotzek S, Schadendorf D, Rassaf T (2018) Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: a meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol 25:482–494. https://doi.org/10.1177/2047487318755193
Totzeck M, Mincu RI, Rassaf T (2017) Cardiovascular adverse events in patients with cancer treated with bevacizumab: a meta-analysis of more than 20,000 patients. J Am Heart Assoc. https://doi.org/10.1161/jaha.117.006278
Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T (2019) Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol 280:163–175. https://doi.org/10.1016/j.ijcard.2019.01.038
Triguero-Cánovas D, López-Rodríguez-Arias F, Gómez-Martínez M, Sánchez-Guillén L, Peris-Castelló F, Alcaide-Quirós MJ, Morillas-Blasco P, Arroyo A, Ramírez JM (2023) Home-based prehabilitation improves physical conditions measured by ergospirometry and 6MWT in colorectal cancer patients: a randomized controlled pilot study. Support Care Cancer 31:673. https://doi.org/10.1007/s00520-023-08140-4
Tu H, Wen CP, Tsai SP, Chow WH, Wen C, Ye Y, Zhao H, Tsai MK, Huang M, Dinney CP, Tsao CK, Wu X (2018) Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ 360:k134. https://doi.org/10.1136/bmj.k134
Vainshtein A, Tryon LD, Pauly M, Hood DA (2015) Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Physiol Cell Physiol 308:C710-719. https://doi.org/10.1152/ajpcell.00380.2014
van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, de Maaker-Berkhof M, Boven E, Schrama J, Geenen MM, Meerum Terwogt JM, van Bochove A, Lustig V, van den Heiligenberg SM, Smorenburg CH, Hellendoorn-van Vreeswijk JA, Sonke GS, Aaronson NK (2015) Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol 33:1918–1927. https://doi.org/10.1200/jco.2014.59.1081
Waissengein B, Abu Ata B, Merimsky O, Shamai S, Wolf I, Arnold JH, Bar-On T, Banai S, Khoury S, Laufer-Perl M (2023) The predictive value of high sensitivity troponin measurements in patients treated with immune checkpoint inhibitors. Clin Res Cardiol 112:409–418. https://doi.org/10.1007/s00392-022-02118-8
Wallen MP, Hennessy D, Brown S, Evans L, Rawstorn JC, Wong Shee A, Hall A (2020) High-intensity interval training improves cardiorespiratory fitness in cancer patients and survivors: a meta-analysis. Eur J Cancer Care (Engl) 29:e13267. https://doi.org/10.1111/ecc.13267
Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal-Ginard B, Torella D, Ellison GM (2014) The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J 35:2722–2731. https://doi.org/10.1093/eurheartj/ehs338
Wernhart S, Balcer B, Rassaf T, Luedike P (2023) Increased dead space ventilation as a contributing factor to persistent exercise limitation in patients with a left ventricular assist device. J Clin Med. https://doi.org/10.3390/jcm12113658
Wernhart S, Goertz A, Hedderich J, Papathanasiou M, Hoffmann J, Rassaf T, Luedike P (2023) Diastolic exercise stress testing in heart failure with preserved ejection fraction: The DEST-HF study. Eur J Heart Fail 25:1768–1780. https://doi.org/10.1002/ejhf.2995
Wernhart S, Hedderich J, Wunderlich S, Schauerte K, Weihe E, Dellweg D, Siemon K (2021) The feasibility of high-intensity interval training in patients with intensive care unit-acquired weakness syndrome following long-term invasive ventilation. Sports Med Open 7:11. https://doi.org/10.1186/s40798-021-00299-6
Wernhart S, Mincu R, Balcer B, Rammos C, Muentjes C, Rassaf T (2023) The cardiorespiratory optimal point as a discriminator of lesion severity in adults with congenital heart disease. J Sports Med Phys Fitness 63:941–948. https://doi.org/10.23736/s0022-4707.23.14835-3
Wernhart S, Oster M, Schulze M, Papathanasiou M, Ruhparwar A, Rassaf T, Luedike P (2023) Moderate continuous and modified high-intensity interval training in patients with left ventricular assist devices: the prospective train-the-LVAD trial. J Card Fail 29:841–848. https://doi.org/10.1016/j.cardfail.2023.01.007
Wernhart S, Papathanasiou M, Jakstaite A, Hoffmann J, Schmack B, Hedderich J, Ruhparwar A, Rassaf T, Luedike P (2023) Exercise oscillatory ventilation in patients with advanced heart failure with and without left ventricular assist device. Artif Organs 47:168–179. https://doi.org/10.1111/aor.14398
Wernhart S, Papathanasiou M, Mahabadi AA, Rassaf T, Luedike P (2023) Betablockers reduce oxygen pulse increase and performance in heart failure patients with preserved ejection fraction. Int J Cardiol 370:309–318. https://doi.org/10.1016/j.ijcard.2022.10.009
Wernhart S, Papathanasiou M, Rassaf T, Luedike P (2023) The controversial role of beta-blockers in heart failure with preserved ejection fraction. Pharmacol Ther 243:108356. https://doi.org/10.1016/j.pharmthera.2023.108356
Wernhart S, Papathanasiou M, Rassaf T, Luedike P (2023) Heart failure classification based on resting ejection fraction does not display a unique exercise response pattern. Int J Cardiol 376:157–164. https://doi.org/10.1016/j.ijcard.2023.01.072
West MA, Lythgoe D, Barben CP, Noble L, Kemp GJ, Jack S, Grocott MP (2014) Cardiopulmonary exercise variables are associated with postoperative morbidity after major colonic surgery: a prospective blinded observational study. Br J Anaesth 112:665–671. https://doi.org/10.1093/bja/aet408
West MA, Parry MG, Lythgoe D, Barben CP, Kemp GJ, Grocott MP, Jack S (2014) Cardiopulmonary exercise testing for the prediction of morbidity risk after rectal cancer surgery. Br J Surg 101:1166–1172. https://doi.org/10.1002/bjs.9551
White E, Mehnert JM, Chan CS (2015) Autophagy, metabolism, and cancer. Clin Cancer Res 21:5037–5046. https://doi.org/10.1158/1078-0432.Ccr-15-0490
WHO (2022) Cancer Key Facts. In:
Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO (2000) Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88:2219–2226. https://doi.org/10.1152/jappl.2000.88.6.2219
Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T (2007) Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115:3086–3094. https://doi.org/10.1161/circulationaha.106.675041
Wonisch M, Hofmann P, Fruhwald FM, Kraxner W, Hödl R, Pokan R, Klein W (2003) Influence of beta-blocker use on percentage of target heart rate exercise prescription. Eur J Cardiovasc Prev Rehabil 10:296–301. https://doi.org/10.1097/00149831-200308000-00013
Yang L, Morielli AR, Heer E, Kirkham AA, Cheung WY, Usmani N, Friedenreich CM, Courneya KS (2021) Effects of exercise on cancer treatment efficacy: a systematic review of preclinical and clinical studies. Cancer Res 81:4889–4895. https://doi.org/10.1158/0008-5472.Can-21-1258
You Y, Lai X, Pan Y, Zheng H, Vera J, Liu S, Deng S, Zhang L (2022) Artificial intelligence in cancer target identification and drug discovery. Signal Transduct Target Ther 7:156. https://doi.org/10.1038/s41392-022-00994-0
Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, Muley A, Asara JM, Paik J, Elemento O, Chen Z, Pappin DJ, Dow LE, Papadopoulos N, Gross SS, Cantley LC (2015) Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 350:1391–1396. https://doi.org/10.1126/science.aaa5004
Zong WX, Rabinowitz JD, White E (2016) Mitochondria and cancer. Mol Cell 61:667–676. https://doi.org/10.1016/j.molcel.2016.02.011
Zucker IH, Musch TI (2018) Benefits of exercise training on cardiovascular dysfunction: molecular and integrative. Am J Physiol Heart Circ Physiol 315:H1027-h1031. https://doi.org/10.1152/ajpheart.00516.2018
Funding
Open Access funding enabled and organized by Projekt DEAL. No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
TR had the idea for the article, SW performed the literature search and data analysis, and drafted the manuscript. TR critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
SW reports no conflicts of interest. TR reports honoraria for lectures and advisory board activities for Bayer, AstraZeneca, Daiichi Sankyo, Pfizer. TR is co-founder of Mycor, a start-up focusing on the development of algorithms to detect amyloidosis. None of these are related to the submitted manuscript.
Ethical standards
The manuscript does not contain clinical studies or patient data.
Consent to participate/publish
Not applicable/both authors have read and approved the final version of the manuscript.
Additional information
This article is part of the special issue “Cardio-Oncology”.
The phrase “topical collection” was changed to “special issue”.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wernhart, S., Rassaf, T. Exercise, cancer, and the cardiovascular system: clinical effects and mechanistic insights. Basic Res Cardiol (2024). https://doi.org/10.1007/s00395-024-01034-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-024-01034-4