Abstract
Cardiovascular and oncological diseases represent the global major causes of death. For both, a novel and far-reaching risk factor has been identified: clonal hematopoiesis (CH). CH is defined as clonal expansion of peripheral blood cells on the basis of somatic mutations, without overt hematological malignancy. The most commonly affected genes are TET2, DNMT3A, ASXL1 and JAK2. By the age of 70, at least 20–50% of all individuals carry a CH clone, conveying a striking clinical impact by increasing all-cause mortality by 40%. This is due predominantly to a nearly two-fold increase of cardiovascular risk, but also to an elevated risk of malignant transformation. Individuals with CH show not only increased risk for, but also worse outcomes after arteriosclerotic events, such as stroke or myocardial infarction, decompensated heart failure and cardiogenic shock. Elevated cytokine levels, dysfunctional macrophage activity and activation of the inflammasome suggest that a vicious cycle of chronic inflammation and clonal expansion represents the major functional link. Despite the apparently high impact of this entity, awareness, functional understanding and especially clinical implications still require further research. This review provides an overview of the current knowledge of CH and its relation to cardiovascular and hematological diseases. It focuses on the basic functional mechanisms in the interplay between atherosclerosis, inflammation and CH, identifies issues for further research and considers potential clinical implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertension, dyslipidemia, diabetes, obesity and smoking are the most common and widely known cardiovascular risk factors. Nevertheless, the global profile of atherosclerosis has changed substantially over the last decade. Given the association of cardiovascular diseases (CVD) with prosperity, worldwide socioeconomic development has spurred the spread of atherosclerosis around the globe. In parallel with the changes in distribution, risk stratification is also undergoing a transformation. Due to the effective and widely affordable administration of cholesterol reducing and anti-hypertensive drugs, risk factors beyond the traditional candidates are gaining impact. Consequentially, this has led to a shift in patient’s risk characteristics (e.g., toward younger age, female sex, normal body mass index [BMI] and different ethnicity). This emphasizes the need to understand atherosclerotic risk beyond metabolic syndrome and other traditional elicitors to prevent disease development in the future [59]. A novel, recently described non-traditional risk factor is a hematopoietic manifestation called CH.
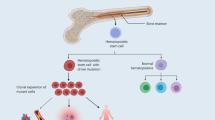
In the broad sense, CH describes any clonal hematopoietic proliferation, including leukemias and other malignant hematological diseases. More narrowly, the term is used for clonal proliferation of hematopoietic cells without overt hematological malignancy. This definition of CH compromises two distinct, major entities: clonal hematopoiesis of indeterminate potential (CHIP) and clonal cytopenia of undetermined significance (CCUS), which is distinguished from CHIP by the presence of cytopenias.
CHIP was first recognized in the 1990s, when Fey et al. observed skewed X-linked inactivation in older women and were the first to propose a potential mechanism of acquired clonality. Later, the same group identified specific somatic mutations in some of the affected women [23, 38] and referred to the condition as being “of indeterminate potential” because of the lack of a clear association to disease. Since then, however, research has provided mounting evidence to the contrary.
During aging, proliferating cells acquire somatic mutations due to stress, exogenous factors and increasing susceptibility to errors during DNA-replication. Most of these mutations are either neutral or detrimental at the single cell level and remain insignificant. Others, if occurring within a particular driver gene or regulatory sequence, may bestow a selective advantage and even contribute to malignant transformation. Along this evolutionary process, transitions are fluid and pre-malignant lesions are common for most oncologic entities. Recognition of this has identified valuable opportunities for screening approaches and pre-emptive treatment before disease development. However, contrary to other pre-malignant entities, CHIP conveys much broader systemic impact not limited to the hematopoietic system. In particular, it has been shown to be associated with increased all-cause mortality and cardiovascular risk [54, 55].
Given the clinical significance as a pre-malignant state and cardiovascular risk factor, CHIP is gaining increasing recognition as a distinct and relevant clinical entity. This is leading to discussions about screening and precautionary interventions that draw a unique and promising connection between the hemato-oncological and cardiovascular fields in terms of current and future (preventive) clinical approaches. However, the importance of this phenomenon has not gained enough awareness, yet. A concerted basic and translational research effort will be required to establish standard clinical guidance recommendations. Not least, several crucial questions remain unanswered: How to screen? Who to screen? Who to treat and how to treat?
Definition of CHIP: how to measure?
CHIP is historically defined as the presence of somatic mutations in the peripheral blood with a clonal size of at least 2% variant allele frequency (VAF), in the absence of overt hematological disease [84].
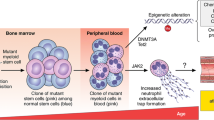
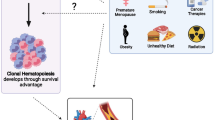
The two most frequently affected genes are DNA (cytosine-5) methyltransferase 3 alpha (DNMT3A) and Tet methylcytosine dioxygenase 2 (TET2), followed by additional sex combs-like 1 (ASXL1) and serine/arginine-rich splicing factor 2 (SRSF2), Janus kinase 2 (JAK2) and Cbl proto-oncogene (CBL), among others [55]. These genes mostly encode for proteins involved in epigenetic regulation, such as DNA-methylation (TET2, DNMT3A) or histone modification (ASXL1), or for spliceosome constituents (SRSF2, SF3B1) or signaling proteins (JAK2, CBL, GNB1, GNAS) [8]. In contrast to established myeloid malignancies, such as acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS), CHIP clones tend to be characterized by a more restricted range of mutations as well as a smaller clone size [8]. The predominant mutation pattern in CHIP is a single substitution of cytosine to thymidine, mostly due to spontaneous deamination. This is considered to be a signature of aging, as DNA error correction decreases in efficiency. It is therefore not surprising that age represents the strongest risk factor for CHIP [87]. By the age of 70, CHIP can be identified in 20–50% of all individuals depending on the sensitivity and gene coverage of the assay used to identify clonal variants [22, 46, 55]. Recent studies that use genome-wide approaches to detect clones that lack mutations in ‘known drivers’ of myeloid neoplasia, even suggest CH to be near-ubiquitous in the aging hematopoietic system, the reported prevalence of CH being limited only by the detection method used [5, 90, 94]. Besides age, some other factors predispose for developing CHIP (Fig. 1), such as previous oncologic treatment [16, 29, 50], male sex [43], smoking [55], unhealthy diet [11] and specific changes in microbiome composition [68]. Furthermore, chronic inflammatory diseases, such as auto-inflammation [7, 80, 96] or HIV-infection [33] seem to favor the emergence of CHIP (Fig. 1). The potential relevance of genetic predisposition remains contentious. Some studies point out a familial predisposition [48, 98] but this has not been supported by twin studies [20, 37, 44].
Risk factors and associated diseases of CH. Several circumstances (left) have been shown to promote the emergence of CH, with age representing the strongest risk factor. Regarding a genetic predisposition, current data remain contentious (empty arrow). However, CH is associated with an increasing number of diseases (right). Most of these are cardiac or malignant conditions. Nonetheless, associations with metabolic and chronic inflammatory disease have also been recently identified
Although CHIP is associated with an increase in red cell distribution width [42], there is, by definition, no significant change in blood cell counts. This makes genetic analyses essential for screening and diagnosing CHIP. Since the reported prevalence of CHIP is strongly dependent on the sensitivity of the sequencing assay employed [53, 90], it is important to define the basic conditions for potential screening approaches.
Current definitions of CHIP, such as that adopted in the most recent 5th edition of the WHO classification of hematolymphoid tumors, require detection of a variant in a known set of driver genes. However, it has recently become clear first, that expansion of clones with no such driver variants is common in the aging hematopoietic system [43, 69, 74, 98] and second, that CH without involvement of a known driver gene seems nonetheless to be associated with an increase in all-cause life-time mortality risk comparable to that of CHIP with a known driver [98].
CHIP: a new cardiovascular risk factor
Independent of traditional cardiovascular risk factors, CHIP was shown to nearly double the cardiovascular risk compared to healthy individuals, thereby establishing CHIP as a new and persuasive cardiovascular risk factor. The individual risk increases with the size of the particular clone as represented by the VAF, which can vary between 2% (lower definition of CHIP) and 50% (equivalent to all cells carrying a heterozygous mutation) [42, 54, 55]. In the meantime, however, there is increasing evidence that CH clones well below 2% VAF can also impact cardiovascular life time [13, 54, 55].
Jaiswal et al. were the first to draw the connection of CHIP with CVD. Moreover, they provided a functional explanation. They exposed TET2-deficient and LDL-receptor defective mice, established by competitive bone marrow transplantation, to a high fat diet and observed a severe enlargement of atherosclerotic plaques [42, 54]. Similarly, an atherogenic diet of Jak2-mutant mice led to larger and more complex atherosclerotic plaques with larger necrotic cores, earlier lesion formation and greater pro-inflammatory immune cell activation compared to wild type mice [89].
Since the original identification of CHIP as a potent cardiovascular risk factor, several retrospective studies have further elucidated the connection between CHIP and common cardiovascular risk factors. For instance, CHIP occurs more frequently in smokers [16, 29, 31, 45, 49, 58]. Since tobacco is a mutagenic agent, the implication is clear. However, functional experiments identifying a direct causal effect are still lacking. There does seem to be a link between CHIP and metabolic imbalance, based on retrospective and in vitro data [18, 34, 41]. Cohort-analyses show that the presence of CHIP increases the likelihood of developing diabetes mellitus by 30% [18, 55]. Surprisingly, no associations were observed regarding lipid profiles, except for JAK2-mutations, which are negatively associated with total cholesterol and LDL cholesterol. Mechanistically, TET2 is involved in regulation of the cellular metabolic homeostasis through AMP-activated protein kinase (AMPK), which represents a central cellular sensor of metabolic demand. In this context, hyperglycemia could promote a clonal advantage in TET2 mutated stem cells [34]. In parallel, other groups have shown that depletion of TET2 might accelerate atherosclerosis due to dysfunctional autophagy, as a major AMPK-regulated pathway [60, 97].
Current evidence for clinical relevance of CHIP
Atherosclerosis and myocardial infarction
In 2014 Jaiswal et al. were the first to describe a statistical association between atherosclerotic events and the presence of CHIP with a hazard ratio of 2.0 (95% confidence interval [CI] 1.1–1.8) for coronary artery disease (CAD) [55]. In a subsequent study, this group later compared 4726 CHIP-patients to a control group of 3529 individuals and confirmed a 1.9-fold overall risk of developing CAD for individuals affected by CHIP. They further measured the coronary artery calcification score based on computer tomography and detected a 3.3-fold increase in CHIP carriers. In line with this, the risk of developing early onset myocardial infarction, (age men < 40 years/woman < 50 years) was four times higher among CHIP-affected individuals. Closer analysis revealed two additional parameters to modulate the cardiovascular risk. First, risk was dependent on the clonal size (VAF). Second, it was dependent on the identity of the affected gene with mutations in JAK2 leading to a 12-fold increase, whereas mutations in the most frequently affected genes TET2 and DNMT3A conferred a two-fold increase in the risk of developing atherosclerosis [54]. The association between CHIP and CAD was replicated in a cohort of postmenopausal women where the likelihood was increased by a factor of 1.36 (95% CI 1.07–1.73) [49]. Since then, further clinical data have emerged from a range of CVDs (Table 1).
Stroke
In line with the overall increased cardiovascular risk, the presence of a CHIP mutation was associated with a 2.6-fold increased likelihood for ischemic stroke in the cohort analyzed by Jaiswal et al. [55]. This increased risk of total strokes was replicated in a further cohort, following adjustment for age, sex and ethnicity, with a hazard ratio of 1.14 (95% CI 1.03–1.2). Importantly, the risk for hemorrhagic stroke was increased 1.24-fold, with TET2 being the strongest risk associated gene. Overall, CHIP-bearers were prone to hemorrhagic rather than to ischemic stroke. Among ischemic strokes, TET2 again showed the strongest association, with microvascular pathology being more frequent than large-vessel strokes [11].
Heart failure
The outcomes of patients with heart failure (HF) has repeatedly been associated with CHIP [9, 30, 35, 56, 70, 71], with correlations resembling those in atherosclerosis. Namely, the HF risk increases with clone VAF [9, 35, 56, 95] and with the number of the acquired mutations [30].
Atherosclerosis represents the major cause of congestive HF [12] and CHIP-carriers confer a significantly increased HF-associated mortality after having experienced myocardial infarction [30, 35]. Inflammation appears to lay an important role here [61]. In mouse models lacking TET2 or DNMT3A, blockage of Interleukin-1β (IL-1b) rescued the deficiency in heart function [78, 79]. Furthermore, transcriptome analysis of HF patients with DNMT3A mutations reveal elevated expression levels of inflammatory genes [4]. Hence, one possibility might be that CHIP is associated with HF through inflammation-mediated effects on atherosclerosis. Nevertheless, in the cohort of Dorsheimer et al., most of the deaths among TET2 or DNMT3A-mutation carriers were attributed to arrhythmic events or progression of HF rather than to myocardial infarction [35]. Furthermore, a large meta-analysis including over 55,000 participants failed to identify any difference between CHIP carrying HF patients regarding prior CAD [95]. Sano et al. investigated the pathogenesis of HF upon CHIP-mutations by establishing a mouse model system. For this purpose, they infused angiotensin II to mimic hypertensive HF and observed increased cardiac dysfunction associated with lack of TET2 or of DNMT3A. Interestingly, the phenotype induced by loss of TET2 or DNMT3A was accompanied by increased cytokine release and expansion of the hematopoietic compartment [79].
Cardiogenic shock represents the most severe clinical manifestation of acute heart failure. Böhme et al. recently studied the prognostic impact of CHIP in 446 patients with cardiogenic shock in acute myocardial infarction from the CULPRIT-SHOCK randomized clinical trial [15]. CHIP variants at ≥ 2% VAF were found in 29% of the patient population, most frequently in the DNMT3A and TET2 genes. Compared to non-CHIP patients, CHIP carriers experienced worse short-term clinical outcome with regard to a composite endpoint of all-cause mortality and the need for renal replacement therapy, even after adjustment for traditional risk factors and several established biomarkers such as arterial lactate, NT pro brain natriuretic peptide (NT-proBNP), cystatin C or IL-6. In line with this, Scolari et al. observed a 1.5-fold increased prevalence of CHIP-mutations, particularly TET2 and ASXL1, among patients with cardiogenic shock predominantly of non-ischemic origin, compared to HF patients (95% CI 1.0–2.1). At the same time, harboring a CHIP mutation was associated with a decrease of survival at multiple timepoints (30-days: HR 2.7; 95% CI 1.3–5.7, P = 0.006; 90-days: HR 2.2; 95% CI 1.3–3.9, P = 0.003; and 3-years: HR 1.7; 95% CI 1.1–2.8, P = 0.01), while individuals carrying a TET2-mutation displayed elevated serum levels of SCD40L, IFNγ, IL-4 and TNFα [81].
Aortic stenosis and other conditions
Sequencing a cohort of 279 patients with aortic valve stenosis (AS) having undergone transcatheter aortic valve intervention (TAVI) identified another association with CHIP. Mortality between one and eight months after TAVI was increased for carriers of DNMT3A or TET2 by a factor of 3.1 (95% CI 1.17–8.08) after adjustment for sex and age, and by 4.8-fold (95% CI 1.49–15.57) after further adjustment for NT-proBNP serum levels. Interestingly, basic clinical parameters such as concomitant atherosclerotic disease, blood cell count, inflammatory markers or procedural characteristics were comparable between CHIP and non-CHIP patients [66]. Another study analyzed monocytes of AS patients carrying CHIP-mutations and detected an increased expression of inflammatory genes and mediators, including IL-1b, IL-6-receptor, NLRP3 inflammasome complex and CD163 [4].
Compared to the other common CHIP mutations, those in JAK2 carry a specific risk profile. Wolach et al. identified a 12-fold increased risk for venous thrombosis among JAK2-mutation carriers, compared to a doubled risk among the other CHIP mutations. The authors offered mechanistical insight using Jak2V617F knockin mouse model, in which increased formation of neutrophilic extracellular traps, as a mechanism of innate immunity, promoted thrombosis [91]. However, an independent study analyzed a cohort of 61 patients with unprovoked pulmonary embolism, 12 of whom carried CHIP-mutations, and failed to detect any difference between CHIP carriers and non-CHIP carriers. Possibly due to the small number of CHIP-positive individuals, no JAK2 mutation was found [82].
Another association with CHIP, and in particular with TET2 mutation, was recently demonstrated for pulmonary arterial hypertension (PAH) within a cohort of 1832 individuals. In this case, contradictory to the aforementioned data, the identification of a TET2 mutation predicted a favorable course of disease, with disease onset being delayed and pulmonary artery pressure being lower among TET2 mutation carriers. Nonetheless, the group found in accompanying mouse experiments that depletion of Tet2 did provoke PAH and this effect was reversible by blockage of IL-1b [75].
Mechanisms of increased CV risk in persons with CH
Macrophage activity and cytokine release
Atherosclerosis represents a severe vascular pathology, driven by the interplay between dyslipidemia and inflammation (Fig. 2). Given the evidence discussed above, it seems likely that CHIP promotes a pro-inflammatory environment, including changes of monocyte and macrophage biology, implying that chronic inflammation might embody the portentous link between cardiovascular risk and CHIP.
Functional interconnections between CHIP and CVD. Somatic mutations within CHIP-associated genes contribute to atherosclerotic pathomechanisms at various stages. Possible interferences of CHIP and atherogenesis are depicted in yellow. Effects appear gene-specific. Notably, some mutations may lead to loss (as assumed for most TET2-mutations or the hotspot-mutation R822H within DNMT3A) and others to gain of function, which raises a special challenge in the search for common mechanisms of CHIP-associated risk increases. sm smooth muscle cell, ICAM-1 Intercellular Adhesion Molecule 1, VCAM-1 vascular cell adhesion protein 1, ABCA ATP-binding cassette transporter A1
This conclusion was based initially on the observation of significantly increased cytokine levels in association with CHIP mutations. A direct causal association between CHIP and atherosclerosis via inflammation was subsequently demonstrated by Fuster et al. [41], who observed increased levels of IL-1b and an apparent atherosclerotic phenotype in Tet2 mutant mice. Blockage of IL-1b diminished the arteriosclerotic phenotype, identifying inflammation as the crucial connection [42]. Amelioration of atherosclerosis and HF in Tet2 depleted mice by pharmacological inhibition of the NLRP3 inflammasome (MCC950), followed by reduction of IL-1b further confirmed this functional concept [79].
These cytokines strongly regulate monocyte rolling, adhesion and transmigration through endothelium, followed by macrophage transformation, all of which are key processes contributing to atherosclerosis [59]. Global changes in cytokine levels and skewed monocytic and macrophagic functions therefore represent a compelling elicitor for the vascular pathology.
The majority of research in this area has analyzed the effects of variants in the two genes most frequently mutated in CH: TET2 and DNMT3A. As described above, TET2-depletion resulted in increased release of the cytokines IL-1b, IL-6 and tumor necrosis factor-alpha (TNF-α) in vitro [1, 42, 51, 78], as well as in mouse models [42]. Similar observations were made for DNMT3A. For instance, CRISPR/Cas-9 mediated DNMT3A knockdown provoked elevated levels of the cytokines CXCL1, CXCL2, IL-6 and CCL5 in macrophages [78]. Furthermore, monocytes bearing DNMT3A mutations showed an increased expression of pro-inflammatory genes, including the aforementioned cytokines [3, 14, 61]. In line with these in vitro data, CHIP-carriers also displayed elevated serum levels of IL-6 [14, 28], IL-8 [54], and IL1-b [14].
While modified global cytokine levels apparently represent a major hallmark in terms of CHIP mediated pathomechanism, there also appear to be changes in specific monocytic and macrophagic functions, although this effect seems to be context specific [27]. For instance, patients with germline variants of DNMT3A, showed reduced levels of monocyte secreted IL-10 [67]. In another experiment, DNMT3A stimulated antiviral immune response of macrophages through activating histone deacetylase 9 [64]. In contrast, monocyte-function appeared to remain mainly stable in the presence of TET2 mutations [56], with the exception of the promotion of macrophage migration inhibitory factor (MIF), a pivotal factor for monocytic differentiation, that is characteristically elevated in atherosclerosis [76]. With respect to Jak2-mutants, alterations in macrophage function also appear to contribute to atherosclerosis [89]. Here, it has been shown experimentally that Jak2 mutant macrophages induce DNA replication stress, activate the AIM2 inflammasome and thereby aggravated atherosclerosis [39].
Taking the pro-inflammatory state in atherosclerosis into account, it is not surprising that elevated levels of high-sensitive C-reactive protein (hs-CRP), a non-specific indicator of inflammation, are observed [59]. However, with regards to CHIP contradictory data exist. Some studies found no association of CHIP and hs-CRP [14, 59], whereas others state a significant elevation [21]. Altogether, CHIP appears to disturb the strongly cytokine regulated equilibrium of macrophage expansion and function, which further promotes the development of atherosclerotic lesions.
Lipoprotein- and cellular metabolism
Lipoprotein homeostasis is a further factor contributing to the formation of atherosclerosis. Macrophages that arise from transmigrated circulating monocytes are able to ingest oxidized or native LDL inside the atherosclerotic lesion. If this process is dysfunctional or macrophages become overloaded, they can turn into foam cells and contribute to arteriosclerotic lesion progression [59]. Just recently, CHIP was found to have an impact in this process. Dotan et al. investigated mutant Jak2 mice and macrophages. They observed attenuated cholesterol efflux from macrophages, most likely due to dysfunctional ABCA1 cholesterol transporter, while cholesterol uptake was unaffected, leading to the acceleration of atherosclerosis. Consistent with this, systemic inhibition of JAK2 with ruxolitinib provoked the same effect [36]. In large CHIP cohorts, however, no alterations of serum cholesterol and its derivates were detected [14, 36, 54, 55].
One of the major hubs to sense and regulate cellular energy and substrate supply is the ubiquitous and highly conserved process of autophagy. Macroautophagic degradation is thought to accelerate atherosclerosis at several breaking points, such as efferocytosis, lipid metabolism and oxidative homeostasis of the endothelium [62]. THP1-derived macrophages, that were incubated with Ox-LDL, showed an attenuation of their macrophagic activity in a TET2 dependent manner, and effect that was reversible by mimicking de-methylation through treatment with azacytidine [60]. Immunohistochemical staining of aortic walls in mice revealed both reduced expression of Tet2 and reduced autophagic activity upon low shear stress. Changes in the expression of TET2 were shown to regulate autophagic activity in endothelial cell cultures, with the knockdown of Tet2 being accompanied by decreased expression of endothelial NO-synthase and increased expression of endothelin-1 as a typical hallmark of endothelial dysfunction [93]. Where it is of note that a recent report of endothelial cell differentiation form circulating monocytoid cells in a sheep model raises the possibility that CHIP myeloid cells may have the potential to transdifferentiate and contribute directly to the endothelial compartment [11]. In ApoE−/− mice, overexpression of Tet2 was followed by upregulation of autophagy reduced atherosclerotic lesions and a decrease in the expression of ICAM-1 and VCAM-1, which are crucial for monocyte invasion. Despite this, cholesterol, tri-acyl-glyceride, and lipoprotein levels remained unchanged [72]. Together, these data indicate an involvement of TET2 in the regulation of autophagy in macrophages and endothelial cells. A potential mediator in this respect is Beclin-1, which is central to the assembly of autophagosomes and the expression of which is controlled by TET2-dependent de-methylation of promotor sequences [72]. It is worth noting in this context that changes in DNA methylation-patterns have widely been associated with increased atherosclerotic risk [6, 63, 73].
In the case of JAK2, further mechanisms have been discussed. As described above, since JAK2 mutations provoke neutrophils to form neutrophil extracellular traps, this may lead to thrombosis, which could further increase CVD risk [91]. Furthermore, Jak2V617F mice showed increased atherosclerotic lesions with complex and comparatively large necrotic cores containing iron, erythrocyte and macrophagic depositions. Reduced erythrocyte expression of CD47 and reduced levels of c-Mer tyrosine kinase (MerTK), as a key modulator of efferocytosis, suggest that impaired efferocytosis and increased erythrophagocytosis may make substantial contributions to the formation of atherosclerotic lesions [89].
Cardio-oncology axis: future perspectives
CHIP mediates a novel and unique bi-directional connection between hematopoiesis and the cardiovascular system, in a functional as well as clinical manner. The exact mechanisms by which some mutations promote a clonal advantage and accelerate atherosclerosis, for instance by provoking inflammation, remain to be elucidated. From what is known so far, CHIP mutations convey enhanced self-renewal of the hematopoietic stem cell (HSC) compartment and concomitantly obstructed hematopoietic differentiation in a mutation-specific fashion [24, 25, 47]. The most frequent mutations within TET2 and DNMT3A further promote granulomonocytic differentiation to the expense of the erythroid lineage [57, 88]. These effects seem to be mediated by the epigenetic enzymatic functions, which coordinate the action of transcription factors regulating self-renewal or myeloid lineage commitment [24]. Notably, the enhancement of HSC-proliferation itself may accelerate CH-development [47]. The currently adopted concept hypothesizes a vicious cycle between enhanced inflammation triggered by CHIP and the emergence of more mutations [52].
Similar mutual associations exist regarding the likelihood of malignant transformation and the prevalence of CHIP among oncologic patients. On the one hand, CHIP carriers bear a higher likelihood of malignant transformation [2, 32, 43], on the other hand radio and chemotherapy increase mutation rate and reduce the stem cell pool, thus increasing proliferative pressure on the remaining cells. This is likely to contribute to the higher prevalence of CHIP among pre-treated oncology patients [29, 65, 92]. Given the strong association of CHIP with increase of cardiovascular life-time risk and the fact that some hematological diseases such as MDS are associated with increased cardiovascular risk anyway [19], this cohort clearly deserves thorough observation or follow-up. With respect to screening approaches, it may be appropriate to routinely subject oncological patients to close surveillance of both cardiovascular function and CHIP mutation status. The presence of a CHIP mutation may ultimately impact treatment decisions.
Potential therapeutic approaches to mitigate CH-associated CV risk
Because of the far-reaching clinical implications of the entity CHIP, there is clearly a case for surveillance and potential preventive intervention. Given the mutual connections between CHIP, malignant disease and CVD, a closely intertwined interdisciplinary communication is likely to be essential for effective clinical management. Having recognized this necessity, some clinics already offer specific CHIP consultation [17, 83]. However, no standard guidance procedures or general recommendations regarding the adequate counseling of affected patients have been established to date. Nonetheless, expert recommendations include a tight monitoring of traditional cardiovascular risk factors, individual risk assessment, adjustment of lifestyle factors and notification of the whole care team. Regarding interventions with the potential of curbing cardiovascular risk, no evidence-based options are available yet, beyond changes in lifestyle and common risk factors. For instance, current data suggests an impact of nutrition, since screening of 48,289 individuals unraveled a higher prevalence of CHIP among those consuming an unhealthy diet, defined as a decreased ratio between healthy elements (fruit and vegetables) to unhealthy elements (red meat, processed food and added salt) [10].
If a clonal somatic mutation is detected in a patient with unexplained cytopenia, further diagnostic testing may be indicated to rule out an underlying overt hematological malignancy. In the event of a dynamic change in blood parameters, a bone marrow puncture should be performed [17].
Nevertheless, screening is only clinically justified if there are potential interventions or pre-emptive treatment options. Initial in vitro experiments have already identified first pharmacological targets (Table 2). One aims to restore TET2’s enzymatic activity by supplementing its cofactor ascorbic acid, which was shown to reverse the enhanced self-renewal of hematopoietic stem and progenitor cells (HSPC) in Tet2-deficient mice [26]. An ongoing clinical trial addresses this relationship by investigating the effect of intravenous high dose ascorbic acid in patients bearing CCUS (NCT03418038), while other clinical approaches target inflammatory processes. Another promising mode of action was examined as part of the CANTOS-trial (Canakinumab Anti-inflammatory Thrombosis Outcome Study). Herein a sub-study observed the effects of the anti-IL-1b antibody Canakinumab on individuals with CHIP [77, 86]. First results indicate a significant reduction of cardiovascular events in patients bearing TET2-mutations [85]. Some trials targeting mutated isocitrate dehydrogenase (IDH) by Enasidenib (NCT05102370) and Ivosidenib (NCT05030441) respectively, are under recruitment. In ASXL-knockin-mice, activation of autophagic activity by blockage of mTOR through rapamycin reversed the phenotype of HSPC expansion [8, 40] thereby identifying a potential future pharmacological point of application.
In conclusion, CH represents a promising field for future containment of cardiovascular risk as well as hematological malignancies. As a prerequisite, further interconnections besides promotion of an inflammatory milieu (such as metabolic and mesenchymal interplay) remain to be elucidated and are likely to reveal further targets for potential pre-emptive treatment options. With respect to HF, the crucial question of the ways in which CHIP, inflammation and atherosclerosis are intertwined in disease evolution and affect prognosis demands further attention. With this goal, this review aims to increase the awareness for CH and point out the areas most relevant for future research. A more thorough appreciation of the diverse interactions and mechanisms can be expected to identify fresh opportunities for translation into the clinic not just for the treatment, but increasingly for the prevention of CVD.
Abbreviations
- AS:
-
Aortic stenosis
- AML:
-
Acute myeloid leukemia
- CAD:
-
Coronary artery disease
- CCUS:
-
Clonal cytopenia of undetermined significance
- CHIP:
-
Clonal hematopoiesis of indetermined potential
- CVD:
-
Cardiovascular disease
- DNMT3A:
-
DNA-methyl-transferase 3A
- IL:
-
Interleukin
- JAK2:
-
Janus kinase 2
- MDS:
-
Myelodysplastic syndrome
- MI:
-
Myocardial infarction
- PAH:
-
Pulmonary artery hypertension
- TAVI:
-
Transcatheter aortic valve intervention
- TNF:
-
Tumor necrosis factor
- VAF:
-
Variant allele frequency
References
Abegunde SO, Buckstein R, Wells RA et al (2018) An inflammatory environment containing TNFα favors Tet2 -mutant clonal hematopoiesis. Exp Hematol 59:60–65. https://doi.org/10.1016/j.exphem.2017.11.002
Abelson S, Collord G, Ng SWK et al (2018) Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 559:400–404. https://doi.org/10.1038/s41586-018-0317-6
Abplanalp WT, Cremer S, John D et al (2021) Clonal hematopoiesis-driver DNMT3A mutations alter immune cells in heart failure. Circ Res 128:216–228. https://doi.org/10.1161/CIRCRESAHA.120.317104
Abplanalp WT, Mas-Peiro S, Cremer S et al (2020) Association of clonal hematopoiesis of indeterminate potential with inflammatory gene expression in patients with severe degenerative aortic valve stenosis or chronic postischemic heart failure. JAMA Cardiol 5:1170–1175. https://doi.org/10.1001/jamacardio.2020.2468
Acuna-Hidalgo R, Sengul H, Steehouwer M et al (2017) Ultra-sensitive sequencing identifies high prevalence of clonal hematopoiesis-associated mutations throughout adult life. Am J Hum Genet 101:50–64. https://doi.org/10.1016/j.ajhg.2017.05.013
Agha G, Mendelson MM, Ward-Caviness CK et al (2019) Blood leukocyte DNA methylation predicts risk of future myocardial infarction and coronary heart disease. Circulation 140:645–657. https://doi.org/10.1161/circulationaha.118.039357
Arends CM, Weiss M, Christen F et al (2019) Clonal hematopoiesis in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Haematologica 105:e264–e267. https://doi.org/10.3324/haematol.2019.223305
Asada S, Kitamura T (2021) Clonal hematopoiesis and associated diseases: a review of recent findings. Cancer Sci 112:3962–3971. https://doi.org/10.1111/cas.15094
Assmus B, Cremer S, Kirschbaum K et al (2021) Clonal haematopoiesis in chronic ischaemic heart failure: prognostic role of clone size for DNMT3A- and TET2-driver gene mutations. Eur Heart J 42:257–265. https://doi.org/10.1093/eurheartj/ehaa845
Bhattacharya R (2020) Improved diet quality is associated with lower prevalence of clonal hematopoiesis of indeterminate potential. Circulation 142:A16686
Bhattacharya R, Zekavat SM, Haessler J et al (2022) Clonal hematopoiesis is associated with higher risk of stroke. Stroke 53:788–797. https://doi.org/10.1161/STROKEAHA.121.037388
Bhattacharya R, Bick AG (2021) Clonal hematopoiesis of indeterminate potential: an expanding genetic cause of cardiovascular disease. Curr Atheroscler Rep 23:66. https://doi.org/10.1007/s11883-021-00966-9
Bick AG, Pirruccello JP, Griffin GK et al (2020) Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation 141:124–131. https://doi.org/10.1161/CIRCULATIONAHA.119.044362
Bick AG, Weinstock JS, Nandakumar SK et al (2020) Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 586:763–768. https://doi.org/10.1038/s41586-020-2819-2
Böhme M, Desch S, Rosolowski M et al (2022) Impact of clonal hematopoiesis in patients with cardiogenic shock complicating acute myocardial infarction. J Am Coll Cardiol 80:1545–1556
Bolton KL, Ptashkin RN, Gao T et al (2020) Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet 52:1219–1226. https://doi.org/10.1038/s41588-020-00710-0
Bolton KL, Gillis NK, Coombs CC et al (2019) Managing clonal hematopoiesis in patients with solid tumors. J Clin Oncol 37:7–11. https://doi.org/10.1200/JCO.18.00331
Bonnefond A, Skrobek B, Lobbens S et al (2013) Association between large detectable clonal mosaicism and type 2 diabetes with vascular complications. Nat Genet 45:1040–1043. https://doi.org/10.1038/ng.2700
Brunner AM, Blonquist TM, Hobbs GS et al (2017) Risk and timing of cardiovascular death among patients with myelodysplastic syndromes. Blood Adv 1:2032–2040. https://doi.org/10.1182/bloodadvances.2017010165
Buscarlet M, Provost S, Zada YF et al (2017) DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood 130:753–762. https://doi.org/10.1182/blood-2017-04-777029
Busque L, Sun M, Buscarlet M et al (2020) High-sensitivity C-reactive protein is associated with clonal hematopoiesis of indeterminate potential. Blood Adv 4:2430–2438. https://doi.org/10.1182/bloodadvances.2019000770
Busque L, Buscarlet M, Mollica L et al (2018) Concise review: age-related clonal hematopoiesis: stem cells tempting the devil. Stem Cells 36:1287–1294. https://doi.org/10.1002/stem.2845
Busque L, Patel JP, Figueroa ME et al (2012) Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet 44:1179–1181. https://doi.org/10.1038/ng.2413
Challen G, Goodell MA (2020) Clonal hematopoiesis: mechanisms driving dominance of stem cell clones. Blood. https://doi.org/10.1182/blood.2020006510
Christen F, Hablesreiter R, Hoyer K et al (2021) Modeling clonal hematopoiesis in umbilical cord blood cells by CRISPR/Cas9. Leukemia. https://doi.org/10.1038/s41375-021-01469-x
Cimmino L, Dolgalev I, Wang Y et al (2017) Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell 170:1079-1095.e20. https://doi.org/10.1016/j.cell.2017.07.032
Cobo I, Tanaka T, Glass CK et al (2021) Clonal hematopoiesis driven by DNMT3A and TET2 mutations: role in monocyte and macrophage biology and atherosclerotic cardiovascular disease. Curr Opin Hematol 29:1–7. https://doi.org/10.1097/MOH.0000000000000688
Cook EK, Izukawa T, Young S et al (2019) Comorbid and inflammatory characteristics of genetic subtypes of clonal hematopoiesis. Blood Adv 3:2482–2486. https://doi.org/10.1182/bloodadvances.2018024729
Coombs CC, Zehir A, Devlin SM et al (2017) Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell 21:374-382.e4. https://doi.org/10.1016/j.stem.2017.07.010
Cremer S, Kirschbaum K, Berkowitsch A et al (2020) Multiple somatic mutations for clonal hematopoiesis are associated with increased mortality in patients with chronic heart failure. Circ Genom Precis Med 13:e003003. https://doi.org/10.1161/circgen.120.003003
Dawoud AAZ, Tapper WJ, Cross NCP (2020) Clonal myelopoiesis in the UK Biobank cohort: ASXL1 mutations are strongly associated with smoking. Leukemia 34:2660–2672. https://doi.org/10.1038/s41375-020-0896-8
Desai P, Mencia-Trinchant N, Savenkov O et al (2018) Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat Med 24:1015–1023. https://doi.org/10.1038/s41591-018-0081-z
Dharan NJ, Yeh P, Bloch M et al (2021) HIV is associated with an increased risk of age-related clonal hematopoiesis among older adults. Nat Med 27:1006–1011. https://doi.org/10.1038/s41591-021-01357-y
Di Wu, Di Hu, Chen H et al (2018) Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 559:637–641. https://doi.org/10.1038/s41586-018-0350-5
Dorsheimer L, Assmus B, Rasper T et al (2019) Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol 4:25–33. https://doi.org/10.1001/jamacardio.2018.3965
Dotan I, Yang J, Ikeda J et al (2022) Macrophage Jak2 deficiency accelerates atherosclerosis through defects in cholesterol efflux. Commun Biol 5:132. https://doi.org/10.1038/s42003-022-03078-5
Fabre MA, McKerrell T, Zwiebel M et al (2020) Concordance for clonal hematopoiesis is limited in elderly twins. Blood 135:269–273. https://doi.org/10.1182/blood.2019001807
Fey MF, Liechti-Gallati S, von Rohr A et al (1994) Clonality and X-inactivation patterns in hematopoietic cell populations detected by the highly informative M27 beta DNA probe. Blood 83:931–938. https://doi.org/10.1182/blood.V83.4.931.931 (see comments)
Fidler TP, Xue C, Yalcinkaya M et al (2021) The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 592:296–301. https://doi.org/10.1038/s41586-021-03341-5
Fujino T, Goyama S, Sugiura Y et al (2021) Mutant ASXL1 induces age-related expansion of phenotypic hematopoietic stem cells through activation of Akt/mTOR pathway. Nat Commun. https://doi.org/10.1038/s41467-021-22053-y
Fuster JJ, Zuriaga MA, Zorita V et al (2020) TET2-loss-of-function-driven clonal hematopoiesis exacerbates experimental insulin resistance in aging and obesity. Cell Rep 33:108326. https://doi.org/10.1016/j.celrep.2020.108326
Fuster JJ, MacLauchlan S, Zuriaga MA et al (2017) Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355:842–847. https://doi.org/10.1126/science.aag1381
Genovese G, Kähler AK, Handsaker RE et al (2014) Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. NEJM 371:2477–2487. https://doi.org/10.1056/NEJMoa1409405
Hansen JW, Pedersen DA, Larsen LA et al (2020) Clonal hematopoiesis in elderly twins: concordance, discordance, and mortality. Blood 135:261–268. https://doi.org/10.1182/blood.2019001793
Haring B, Reiner AP, Liu J et al (2021) Healthy lifestyle and clonal hematopoiesis of indeterminate potential: results from the women’s health initiative. J Am Heart Assoc 10:e018789. https://doi.org/10.1161/JAHA.120.018789
Hecker JS, Hartmann L, Rivière J et al (2021) CHIP and hips: clonal hematopoiesis is common in patients undergoing hip arthroplasty and is associated with autoimmune disease. Blood 138:1727–1732. https://doi.org/10.1182/blood.2020010163
Heyde A, Rohde D, McAlpine CS et al (2021) Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell 184:1348-1361.e22. https://doi.org/10.1016/j.cell.2021.01.049
Hinds DA, Barnholt KE, Mesa RA et al (2016) Germ line variants predispose to both JAK2 V617F clonal hematopoiesis and myeloproliferative neoplasms. Blood 128:1121–1128. https://doi.org/10.1182/blood-2015-06-652941
Honigberg MC, Zekavat SM, Niroula A et al (2021) Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation 143:410–423. https://doi.org/10.1161/CIRCULATIONAHA.120.051775
Hsu JI, Dayaram T, Tovy A et al (2018) PPM1D mutations drive clonal hematopoiesis in response to cytotoxic chemotherapy. Cell Stem Cell 23:700-713.e6. https://doi.org/10.1016/j.stem.2018.10.004
Ichiyama K, Chen T, Wang X et al (2015) The methylcytosine dioxygenase Tet2 Promotes DNA demethylation and activation of cytokine gene expression in T cells. Immunity 42:613–626. https://doi.org/10.1016/j.immuni.2015.03.005
Jaiswal S, Libby P (2020) Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol 17:137–144. https://doi.org/10.1038/s41569-019-0247-5
Jaiswal S, Ebert BL (2019) Clonal hematopoiesis in human aging and disease. Science. https://doi.org/10.1126/science.aan4673
Jaiswal S, Natarajan P, Silver AJ et al (2017) Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. NEJM 377:111–121. https://doi.org/10.1056/NEJMoa1701719
Jaiswal S, Fontanillas P, Flannick J et al (2014) Age-related clonal hematopoiesis associated with adverse outcomes. NEJM 371:2488–2498. https://doi.org/10.1056/NEJMoa1408617
Kiefer KC, Cremer S, Pardali E et al (2021) Full spectrum of clonal haematopoiesis-driver mutations in chronic heart failure and their associations with mortality. ESC Heart Fail 8:1873–1884. https://doi.org/10.1002/ehf2.13297
Kunimoto H, Nakajima H (2021) TET2: a cornerstone in normal and malignant hematopoiesis. Cancer Sci 112:31–40. https://doi.org/10.1111/cas.14688
Levin MG, Nakao T, Zekavat SM et al (2022) Genetics of smoking and risk of clonal hematopoiesis. Sci Rep 12:7248. https://doi.org/10.1038/s41598-022-09604-z
Libby P (2021) The changing landscape of atherosclerosis. Nature 592:524–533. https://doi.org/10.1038/s41586-021-03392-8
Li G, Peng J, Liu Y et al (2015) Oxidized low-density lipoprotein inhibits THP-1-derived macrophage autophagy via TET2 down-regulation. Lipids 50:177–183. https://doi.org/10.1007/s11745-014-3977-5
Lim GB (2020) Clonal haematopoiesis induces a pro-inflammatory monocyte phenotype in HF. Nat Rev Cardiol 18:74. https://doi.org/10.1038/s41569-020-00481-5
Lin L, Zhang M-X, Zhang L et al (2021) Autophagy, pyroptosis, and ferroptosis: new regulatory mechanisms for atherosclerosis. Front Cell Dev Biol 9:809955. https://doi.org/10.3389/fcell.2021.809955
Lin Z, Ding Q, Li X et al (2021) Targeting epigenetic mechanisms in vascular aging. Front Cardiovasc Med 8:806988. https://doi.org/10.3389/fcvm.2021.806988
Li X, Zhang Q, Ding Y et al (2016) Methyltransferase Dnmt3a upregulates HDAC9 to deacetylate the kinase TBK1 for activation of antiviral innate immunity. Nat Immunol 17:806–815. https://doi.org/10.1038/ni.3464
Loh P-R, Genovese G, Handsaker RE et al (2018) Insights into clonal haematopoiesis from 8342 mosaic chromosomal alterations. Nature 559:350–355. https://doi.org/10.1038/s41586-018-0321-x
Mas-Peiro S, Hoffmann J, Fichtlscherer S et al (2020) Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur Heart J 41:933–939. https://doi.org/10.1093/eurheartj/ehz591
Mba Medie F, Sharma-Kuinkel BK, Ruffin F et al (2019) Genetic variation of DNA methyltransferase-3A contributes to protection against persistent MRSA bacteremia in patients. Proc Natl Acad Sci U S A 116:20087–20096. https://doi.org/10.1073/pnas.1909849116
Meisel M, Hinterleitner R, Pacis A et al (2018) Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature 557:580–584. https://doi.org/10.1038/s41586-018-0125-z
Mitchell E, Spencer Chapman M, Williams N et al (2022) Clonal dynamics of haematopoiesis across the human lifespan. Nature 606:343–350. https://doi.org/10.1038/s41586-022-04786-y
Palomo L, Santiago-Vacas E, Pascual-Figal D et al (2021) Prevalence and characteristics of clonal hematopoiesis in heart failure. Rev Esp Cardiol (Engl Ed) 74:996–999. https://doi.org/10.1016/j.rec.2021.05.005
Pascual-Figal DA, Bayes-Genis A, Díez-Díez M et al (2021) Clonal hematopoiesis and risk of progression of heart failure with reduced left ventricular ejection fraction. J Am Coll Cardiol 77:1747–1759. https://doi.org/10.1016/j.jacc.2021.02.028
Peng J, Yang Q, Li A-F et al (2016) Tet methylcytosine dioxygenase 2 inhibits atherosclerosis via upregulation of autophagy in ApoE–/– mice. Oncotarget 7:76423–76436. https://doi.org/10.18632/oncotarget.13121
Pogribny IP, Beland FA (2009) DNA hypomethylation in the origin and pathogenesis of human diseases. Cell Mol Life Sci 66:2249–2261. https://doi.org/10.1007/s00018-009-0015-5
Poon GYP, Watson CJ, Fisher DS et al (2021) Synonymous mutations reveal genome-wide levels of positive selection in healthy tissues. Nat Genet 53:1597–1605. https://doi.org/10.1038/s41588-021-00957-1
Potus F, Pauciulo MW, Cook EK et al (2020) Novel mutations and decreased expression of the epigenetic regulator TET2 in pulmonary arterial hypertension. Circulation 141:1986–2000. https://doi.org/10.1161/circulationaha.119.044320
Pronier E, Imanci A, Selimoglu-Buet D et al (2022) Macrophage migration inhibitory factor is overproduced through EGR1 in TET2low resting monocytes. Commun Biol 5:110. https://doi.org/10.1038/s42003-022-03057-w
Ridker PM, Everett BM, Thuren T et al (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. NEJM 377:1119–1131. https://doi.org/10.1056/NEJMoa1707914
Sano S, Oshima K, Wang Y et al (2018) CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res 123:335–341. https://doi.org/10.1161/circresaha.118.313225
Sano S, Oshima K, Wang Y et al (2018) Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardiol 71:875–886. https://doi.org/10.1016/j.jacc.2017.12.037
Savola P, Kelkka T, Rajala HL et al (2017) Somatic mutations in clonally expanded cytotoxic T lymphocytes in patients with newly diagnosed rheumatoid arthritis. Nat Commun. https://doi.org/10.1038/ncomms15869
Scolari FL, Abelson S, Brahmbhatt DH et al (2022) Clonal haematopoiesis is associated with higher mortality in patients with cardiogenic shock. Eur J Heart Fail. https://doi.org/10.1002/ejhf.2588
Soudet S, Jedraszak G, Evrard O et al (2021) Is hematopoietic clonality of indetermined potential a risk factor for pulmonary embolism? TH Open 5:e338–e342. https://doi.org/10.1055/s-0041-1733856
Steensma DP, Bolton KL (2020) What to tell your patient with clonal hematopoiesis and why: insights from 2 specialized clinics. Blood 136:1623–1631. https://doi.org/10.1182/blood.2019004291
Steensma DP, Bejar R, Jaiswal S et al (2015) Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126:9–16. https://doi.org/10.1182/blood-2015-03-631747
Svensson EC, Madar A, Campbell CD et al (2022) TET2-driven clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA Cardiol 7:521–528. https://doi.org/10.1001/jamacardio.2022.0386
Swirski FK (2017) From clonal haematopoiesis to the CANTOS trial. Nat Rev Cardiol 15:79–80. https://doi.org/10.1038/nrcardio.2017.208
van Zeventer IA, Salzbrunn JB, de Graaf AO et al (2021) Prevalence, predictors, and outcomes of clonal hematopoiesis in individuals aged ≥ 80 years. Blood Adv 5:2115–2122. https://doi.org/10.1182/bloodadvances.2020004062
Venugopal K, Feng Y, Shabashvili D et al (2021) Alterations to DNMT3A in hematologic malignancies. Cancer Res 81:254–263. https://doi.org/10.1158/0008-5472.CAN-20-3033
Wang W, Liu W, Fidler T et al (2018) Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 V617F mice. Circ Res 123:e35–e47. https://doi.org/10.1161/circresaha.118.313283
Watson CJ, Papula AL, Poon GYP et al (2020) The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 367:1449–1454. https://doi.org/10.1126/science.aay9333
Wolach O, Sellar RS, Martinod K et al (2018) Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aan8292
Wong TN, Miller CA, Jotte MRM et al (2018) Cellular stressors contribute to the expansion of hematopoietic clones of varying leukemic potential. Nat Commun 9:455. https://doi.org/10.1038/s41467-018-02858-0
Yang Q, Li X, Li R et al (2016) Low shear stress inhibited endothelial cell autophagy through TET2 downregulation. Ann Biomed Eng 44:2218–2227. https://doi.org/10.1007/s10439-015-1491-4
Young AL, Challen GA, Birmann BM et al (2016) Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. https://doi.org/10.1038/ncomms12484
Yu B, Roberts MB, Raffield LM et al (2021) Supplemental association of clonal hematopoiesis with incident heart failure. J Am Coll Cardiol 78:42–52. https://doi.org/10.1016/j.jacc.2021.04.085
Zhang CR, Nix D, Gregory M et al (2019) Inflammatory cytokines promote clonal hematopoiesis with specific mutations in ulcerative colitis patients. Exp Hematol 80:36-41.e3. https://doi.org/10.1016/j.exphem.2019.11.008
Zhang J, Tan P, Guo L et al (2019) p53-dependent autophagic degradation of TET2 modulates cancer therapeutic resistance. Oncogene 38:1905–1919. https://doi.org/10.1038/s41388-018-0524-5
Zink F, Stacey SN, Norddahl GL et al (2017) Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 130:742–752. https://doi.org/10.1182/blood-2017-02-769869
Funding
Open Access funding enabled and organized by Projekt DEAL. The author(s) acknowledge support from the German Research Foundation (DFG) and Universität Leipzig within the program of Open Access Publishing.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stein, A., Metzeler, K., Kubasch, A.S. et al. Clonal hematopoiesis and cardiovascular disease: deciphering interconnections. Basic Res Cardiol 117, 55 (2022). https://doi.org/10.1007/s00395-022-00969-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-022-00969-w