Abstract
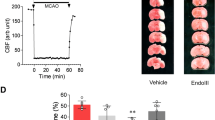
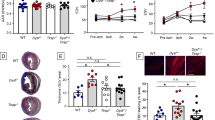
Recent reports indicate that elevating DNA glycosylase/AP lyase repair enzyme activity offers marked cytoprotection in cultured cells and a variety of injury models. In this study, we measured the effect of EndoIII, a fusion protein construct that traffics Endonuclease III, a DNA glycosylase/AP lyase, to the mitochondria, on infarct size in a rat model of myocardial ischemia/reperfusion. Open-chest, anesthetized rats were subjected to 30 min of occlusion of a coronary artery followed by 2 h of reperfusion. An intravenous bolus of EndoIII, 8 mg/kg, just prior to reperfusion reduced infarct size from 43.8 ± 1.4 % of the risk zone in control animals to 24.0 ± 1.3 % with no detectable hemodynamic effect. Neither EndoIII’s vehicle nor an enzymatically inactive EndoIII mutant (K120Q) offered any protection. The magnitude of EndoIII’s protection was comparable to that seen with the platelet aggregation inhibitor cangrelor (25.0 ± 1.8 % infarction of risk zone). Because loading with a P2Y12 receptor blocker to inhibit platelets is currently the standard of care for treatment of acute myocardial infarction, we tested whether EndoIII could further reduce infarct size in rats treated with a maximally protective dose of cangrelor. The combination reduced infarct size to 15.1 ± 0.9 % which was significantly smaller than that seen with either cangrelor or EndoIII alone. Protection from cangrelor but not EndoIII was abrogated by pharmacologic blockade of phosphatidylinositol-3 kinase or adenosine receptors indicating differing cellular mechanisms. We hypothesized that EndoIII protected the heart from spreading necrosis by preventing the release of proinflammatory fragments of mitochondrial DNA (mtDNA) into the heart tissue. In support of this hypothesis, an intravenous bolus at reperfusion of deoxyribonuclease I (DNase I) which should degrade any DNA fragments escaping into the extracellular space was as protective as EndoIII. Furthermore, the combination of EndoIII and DNase I produced additive protection. While EndoIII would maintain mitochondrial integrity in many of the ischemic cardiomyocytes, DNase I would further prevent mtDNA released from those cells that EndoIII could not save from propagating further necrosis. Thus, our mtDNA hypothesis would predict additive protection. Finally to demonstrate the toxicity of mtDNA, isolated hearts were subjected to 15 min of global ischemia. Infarct size doubled when the coronary vasculature was filled with mtDNA fragments during the period of global ischemia. To our knowledge, EndoIII and DNase are the first agents that can both be given at reperfusion and add to the protection of a P2Y12 blocker, and thus should be effective in today’s patient with acute myocardial infarction.







Similar content being viewed by others
References
Bliksøen M, Mariero LH, Ohm IK, Haugen F, Yndestad A, Solheim S, Seljeflot I, Ranheim T, Andersen GØ, Aukrust P, Valen G, Vinge LE (2012) Increased circulating mitochondrial DNA after myocardial infarction. Int J Cardiol 158:132–134. doi:10.1016/j.ijcard.2012.04.047
Boiteux S, Guillet M (2004) Abasic sites in DNA: repair and biological consequences in Saccharomyces cerevisiae. DNA Repair 3:1–12. doi:10.1016/j.dnarep.2003.10.002
Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR (1999) The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res 424:37–49. doi:10.1016/S0027-5107(99)00006-8
Chatterjee A, Mambo E, Zhang Y, DeWeese T, Sidransky D (2006) Targeting of mutant hogg1 in mammalian mitochondria and nucleus: effect on cellular survival upon oxidative stress. BMC Cancer 6:235. doi:10.1186/1471-2407-6-235
Chouteau JM, Obiako B, Gorodnya OM, Pastukh VM, Ruchko MV, Wright AJ, Wilson GL, Gillespie MN (2011) Mitochondrial DNA integrity may be a determinant of endothelial barrier properties in oxidant-challenged rat lungs. Am J Physiol 301:L892–L898. doi:10.1152/ajplung.00210.2011
Cuzzocrea S, Riley DP, Caputi AP, Salvemini D (2001) Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev 53:135–159
Dobson AW, Grishko V, LeDoux SP, Kelley MR, Wilson GL, Gillespie MN (2002) Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am J Physiol 283:L205–L210. doi:10.1152/ajplung.00443.2001
Dobson AW, Xu Y, Kelley MR, LeDoux SP, Wilson GL (2000) Enhanced mitochondrial DNA repair and cellular survival after oxidative stress by targeting the human 8-oxoguanine glycosylase repair enzyme to mitochondria. J Biol Chem 275:37518–37523. doi:10.1074/jbc.M000831200
Fernández-Ruiz I, Arnalich F, Cubillos-Zapata C, Hernández-Jiménez E, Moreno-González R, Toledano V, Fernández-Velasco M, Vallejo-Cremades MT, Esteban-Burgos L, Pérez de Diego R, Llamas-Matias MA, García-Arumi E, Marti R, Boscá L, Andreu AL, López-Sendón JL, López-Collazo E (2014) Mitochondrial DAMPs induce endotoxin tolerance in human monocytes: an observation in patients with myocardial infarction. PLoS One 9:e95073. doi:10.1371/journal.pone.0095073
García N, Chávez E (2007) Mitochondrial DNA fragments released through the permeability transition pore correspond to specific gene size. Life Sci 81:1160–1166. doi:10.1016/j.lfs.2007.08.019
Grishko V, Solomon M, Wilson GL, LeDoux SP, Gillespie MN (2001) Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am J Physiol 280:L1300–L1308
Halestrap AP, Clarke SJ, Javadov SA (2004) Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res 61:372–385. doi:10.1016/S0008-6363(03)00533-9
Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Potter BJ, Wilson GL, Gillespie MN, Parker JC (2013) Mitochondrial-targeted DNA repair enzyme 8-oxoguanine DNA glycosylase 1 protects against ventilator-induced lung injury in intact mice. Am J Physiol 304:L287–L297. doi:10.1152/ajplung.00071.2012
Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Wilson GL, Gillespie MN, Parker JC (2014) Mitochondrial targeted endonuclease III DNA repair enzyme protects against ventilator induced lung injury in mice. Pharmaceuticals 7:894–912. doi:10.3390/ph7080894
Hausenloy DJ, Yellon DM (2003) The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion. J Mol Cell Cardiol 35:339–341. doi:10.1016/S0022-2828(03)00043-9
Heusch G (2013) Cardioprotection: chances and challenges of its translation to the clinic. Lancet 381:166–175. doi:10.1016/S0140-6736(12)60916-7
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. doi:10.1016/0378-1119(89)90358-2
Ibrahim M, Strah H, Scozzi D, Huang HJ, Kreisel D, Krupnick A, Puyo C, Hachem RR, Alouch A, Trulock E, Gelman A (2014) Circulating mitochondrial DNA is elevated in lung transplant recipient patients with immediate early primary graft dysfunction. Am J Respir Crit Care Med 189:A1605
Kloner RA (2013) Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res 113:451–463. doi:10.1161/CIRCRESAHA.112.300627
Koczor CA, Shokolenko IN, Boyd AK, Balk SP, Wilson GL, LeDoux SP (2009) Mitochondrial DNA damage initiates a cell cycle arrest by a Chk2-associated mechanism in mammalian cells. J Biol Chem 284:36191–36201. doi:10.1074/jbc.M109.036020
Konstantinidis K, Kitsis RN (2012) Cardiovascular biology: escaped DNA inflames the heart. Nature 485:179–180. doi:10.1038/485179a
Miki T, Liu GS, Cohen MV, Downey JM (1998) Mild hypothermia reduces infarct size in the beating rabbit heart: a practical intervention for acute myocardial infarction? Basic Res Cardiol 93:372–383. doi:10.1007/s003950050105
Miura T, Ogawa T, Suzuki K, Goto M, Shimamoto K (1997) Infarct size limitation by a new Na+-H+ exchange inhibitor, Hoe 642: difference from preconditioning in the role of protein kinase C. J Am Coll Cardiol 29:693–701. doi:10.1016/S0735-1097(96)00522-0
Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K (2012) Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485:251–255. doi:10.1038/nature10992
Otsuka H, Bhushan S, King AL, Polhemus D, Wilson GL, Lefer DJ (2013) Mitochondrial targeted DNA repair enzyme 8-oxoguanene DNA glycosylase 1 (OGG1) attenuates myocardial infarct size. Circulation 128:A14115
Otsuka H, Bhushan S, King AL, Polhemus D, Wilson GL, Lefer DJ (2013) Mitochondrial targeted DNA repair enzyme, Endonuclease III (ENDO III), attenuates myocardial ischemia/reperfusion injury. Circulation 128:A14149
Panduri V, Liu G, Surapureddi S, Kondapalli J, Soberanes S, de Souza-Pinto NC, Bohr VA, Budinger GRS, Schumacker PT, Weitzman SA, Kamp DW (2009) Role of mitochondrial hOGG1 and aconitase in oxidant-induced lung epithelial cell apoptosis. Free Rad Biol Med 47:750–759. doi:10.1016/j.freeradbiomed.2009.06.010
Pinz KG, Bogenhagen DF (2000) Characterization of a catalytically slow AP lyase activity in DNA polymerase γ and other family A DNA polymerases. J Biol Chem 275:12509–12514. doi:10.1074/jbc.275.17.12509
Rachek LI, Grishko VI, Alexeyev MF, Pastukh VV, LeDoux SP, Wilson GL (2004) Endonuclease III and endonuclease VIII conditionally targeted into mitochondria enhance mitochondrial DNA repair and cell survival following oxidative stress. Nucleic Acids Res 32:3240–3247. doi:10.1093/nar/gkh648
Rachek LI, Grishko VI, LeDoux SP, Wilson GL (2006) Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Rad Biol Med 40:754–762. doi:10.1016/j.freeradbiomed.2005.09.028
Rachek LI, Grishko VI, Musiyenko SI, Kelley MR, LeDoux SP, Wilson GL (2002) Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J Biol Chem 277:44932–44937. doi:10.1074/jbc.M208770200
Ricci C, Pastukh V, Leonard J, Turrens J, Wilson G, Schaffer D, Schaffer SW (2008) Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol 294:C413–C422. doi:10.1152/ajpcell.00362.2007
Roubille F, Lairez O, Mewton N, Rioufol G, Ranc S, Sanchez I, Cung TT, Elbaz M, Piot C, Ovize M (2012) Cardioprotection by clopidogrel in acute ST-elevated myocardial infarction patients: a retrospective analysis. Basic Res Cardiol 107:275. doi:10.1007/s00395-012-0275-3
Ruchko M, Gorodnya O, LeDoux SP, Alexeyev MF, Al-Mehdi A-B, Gillespie MN (2005) Mitochondrial DNA damage triggers mitochondrial dysfunction and apoptosis in oxidant-challenged lung endothelial cells. Am J Physiol 288:L530–L535. doi:10.1152/ajplung.00255.2004
Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L, Brill A, Wang Y, Wagner DD (2014) VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 123:141–148. doi:10.1182/blood-2013-07-514992
Simmons JD, Lee Y-L, Mulekar S, Kuck JL, Brevard SB, Gonzalez RP, Gillespie MN, Richards WO (2013) Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann Surg 258:591–596. doi:10.1097/SLA.0b013e3182a4ea46
Stenslokken KO, Bilksoen M, Mariero L, Ytrehus K, Baysa A, Vaage JI, Valen G (2014) Extracellular mitochondrial DNA induces cell death in cardiomyocytes. Cardiovasc Res 103(Suppl 1):S122. doi:10.1093/cvr/cvu098.93
Tissier R, Hamanaka K, Kuno A, Parker JC, Cohen MV, Downey JM (2007) Total liquid ventilation provides ultra-fast cardioprotective cooling. J Am Coll Cardiol 49:601–605. doi:10.1016/j.jacc.2006.09.041
Torres-Gonzalez M, Gawlowski T, Kocalis H, Scott BT, Dillmann WH (2014) Mitochondrial 8-oxoguanine glycosylase decreases mitochondrial fragmentation and improves mitochondrial function in H9C2 cells under oxidative stress conditions. Am J Physiol 306:C221–C229. doi:10.1152/ajpcell.00140.2013
Wang J, Wang Q, Watson LJ, Jones SP, Epstein PN (2011) Cardiac overexpression of 8-oxoguanine DNA glycosylase 1 protects mitochondrial DNA and reduces cardiac fibrosis following transaortic constriction. Am J Physiol 301:H2073–H2080. doi:10.1152/ajpheart.00157.2011
Yang X-M, Cui L, Alhammouri A, Downey JM, Cohen MV (2013) Triple therapy greatly increases myocardial salvage during ischemia/reperfusion in the in situ rat heart. Cardiovasc Drugs Ther 5:403–412. doi:10.1007/s10557-013-6474-9
Yang X-M, Liu Y, Cui L, Yang X, Liu Y, Tandon N, Kambayashi J, Downey JM, Cohen MV (2013) Platelet P2Y12 blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther 18:251–262. doi:10.1177/1074248412467692
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ (2010) Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464:104–107. doi:10.1038/nature08780
Acknowledgments
This study was funded in part by the National Institutes of Health grants PO1 HL66299 and OD010944 (MA), the National Institute of Environmental Health Sciences grant ES03456 (GW), and Heart, Blood and Lung Institute of the National Institutes of Health grants HL058234 and HL113614 (MNG).
Conflict of interest
GW and MNG are co-founders of Exscien, the manufacturer of Endo III.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, XM., Cui, L., White, J. et al. Mitochondrially targeted Endonuclease III has a powerful anti-infarct effect in an in vivo rat model of myocardial ischemia/reperfusion. Basic Res Cardiol 110, 3 (2015). https://doi.org/10.1007/s00395-014-0459-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-014-0459-0