Abstract
Purpose
High consumption of fruits and vegetables decrease the risk of bladder cancer (BC). The evidence of specific fruits and vegetables and the BC risk is still limited.
Methods
Fruit and vegetable consumptions in relation to BC risk was examined by pooling individual participant data from case–control studies. Unconditional logistic regression was used to estimate study-specific odds ratio’s (ORs) with 95% confidence intervals (CIs) and combined using a random-effects model for intakes of total fruits, total vegetables, and subgroups of fruits and vegetables.
Results
A total of 11 case–control studies were included, comprising 5637 BC cases and 10,504 controls. Overall, participants with the highest intakes versus the lowest intakes of fruits in total (OR 0.79; 95% CI 0.68–0.91), citrus fruits (OR 0.81; 95% CI 0.65–0.98), pome fruits (OR 0.76; 95% CI 0.65–0.87), and tropical fruits (OR 0.84; 95% CI 0.73–0.94) reduced the BC risk. Greater consumption of vegetables in total, and specifically shoot vegetables, was associated with decreased BC risk (OR 0.82; 95% CI 0.68–0.96 and OR 0.87; 95% CI 0.78–0.96, respectively). Substantial heterogeneity was observed for the associations between citrus fruits and total vegetables and BC risk.
Conclusion
This comprehensive study provides compelling evidence that the consumption of fruits overall, citrus fruits, pome fruits and tropical fruits reduce the BC risk. Besides, evidence was found for an inverse association between total vegetables and shoot vegetables intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consumption of fruits and vegetables is an important part of a healthy diet and has been linked to a reduced risk of cancer [1]. A report on diet and bladder cancer (BC) from the WCRF Continuous Update Project concluded that high intakes of fruit and vegetables could decrease the BC risk [2]. In addition, results of a large systematic review suggested that the consumption of citrus fruits and cruciferous vegetables decreases BC risk [3], and another review suggested that a diet rich in vegetables and fruits might be protective against BC [4].
Previous studies have often lacked adequate statistical power to detect associations between dietary factors and BC risk. Aside from total fruit and total vegetable consumption, associations between subgroups of fruits and vegetables and BC risk have been reported sporadically and may be subject to publication bias. As fruits and vegetables are heterogeneous with respect to phytochemical content [5], associations with BC risk may differ between types of fruits and vegetables. The use of individual data from multiple case–control studies could substantially increase statistical power, and covariates can be standardized across studies.
The aim of this large-scale pooled study was to investigate the association between total fruit and total vegetable consumption and specific subgroups of fruits and vegetables and BC risk using data of 5,648 BC cases and 10,517 controls from 11 studies which were included in the Bladder cancer Epidemiology and Nutritional Determinants (BLEND) consortium.
Methods
Study population
Data were analyzed from the BLEND study. BLEND is a large international nutritional consortium on BC, which includes nineteen case–control studies originating from countries all over the world [6]. Eleven of the nineteen case–control studies had sufficient information (i.e., data on usual dietary intake, method of dietary assessment, geographical region, ethnicity, gender, smoking status, disease status, age at BC diagnosis and/or age at enrollment of the study) to be eligible for inclusion in our study on the influence of total fruit and total vegetable consumption and specific subgroups of fruits and vegetables on BC risk. Studies originated from the USA, Belgium, Sweden, Italy, Canada and China. Each participating study has been approved by a local ethics committee. Informed consent was obtained from all individual participants included in each study.
Data collection
Details on the methodology of the BLEND consortium have been described elsewhere [6]. Briefly, BC cases were ascertained by using medical record review or linkage with a cancer registry. Both non-muscle invasive BC (NMIBC) and muscle invasive BC (MIBC) were considered as outcomes.
For each study, participants were asked to report the frequency of fruit and vegetable items consumed during the preceding one or two years before study enrollment. All studies made use of a validated self-administered food frequency questionnaire (FFQ) or a questionnaire administered by a trained interviewer regarding the frequency of fruit and vegetable consumption. Summary details of the FFQs used in the included studies can be found in Appendix 1, Table 5. Total fruit consumption was computed as the sum of all fruit items in each study, and total vegetable consumption as the sum of all vegetable items provided. The following subgroups of fruits were defined: citrus fruits, pome fruits, soft fruits, stone fruits, tropical fruits, fruit mixtures, and fruit products (Table 1). For vegetables, the defined subgroups were: leaf vegetables, brassica, stalk vegetables, shoot vegetables, tubers, onion-family vegetables, root vegetables, fruit vegetables, pod seeds, fungi, seaweeds, vegetables mixtures, and vegetables products (Table 1). No analyses were performed for fruit mixtures, fruit products, onion-family vegetables, root vegetables, fruit vegetables, pod seeds, fungi, seaweeds, vegetable mixtures, or vegetable products because of the limited number of participants consuming fruits or vegetables from these subgroups.
Statistical analysis
Participants who reported a history of cancer other than nonmelanoma skin cancer prior to study entry, or had missing data on age at study entry, gender, smoking status, pack years, or fruit and vegetable consumption, were excluded from the analyses. In the analyses, fruit and vegetable consumption were categorized into three consumption levels (low intakes/moderate intakes/high intakes), corresponding to study-specific marginal tertiles. The investigated fruit subgroups (citrus fruits, pome fruits, soft fruits, stone fruits, and tropical fruits) and vegetable subgroups (leaf vegetables, brassica, stalk vegetables, shoot vegetables, and tubers) were also modeled as study-specific marginal tertiles. Although the tertile approach does not take into account true differences in the distribution of population intakes across studies, reported intakes may differ across studies based on country-specific portions sizes and differences in FFQs used, and is therefore our preferred approach [6].
The analytic approach was a two-stage process. First, study-specific odds ratios (ORs) were calculated using unconditional logistic regression models (with low consumption levels as the reference group). The majority of the included case–control studies matched on age and gender only. Although matched methods (e.g., from conditional logistic regression) are robust to matching distortion, unmatched methods like unconditional logistic regression appear to be viable options for loose-matching data, e.g., data matched on a small number of demographic variables like age and gender. After that, the estimates were pooled using a random effects meta-analysis approach to calculate an overall estimate and 95% confidence interval (CI). Adjustments were made for the following potential confounders: gender (male, female), age at study entry (< 45 years, 45–49 years, 50–54 years, 55–59 years, 60–64 years, 65–69 years, 70–74 years, > 75 years), smoking status (never smoker/former smoker/current smoker), and pack years (< 9 years, 9–17 years, 18–30 years, 31–46 years, > 47 years). Interactions between age and total fruit and vegetable consumption, gender and total fruit and vegetable consumption, and smoking status and total fruit and vegetable consumption were tested and showed no significant interactions. Nonetheless, besides the overall analysis, subgroup analyses were performed on the two main study-specific matching factors gender and age, as recommended by the study of Smith-Warner et al. [7]. Small study effects were analyzed by funnel plots. A sensitivity analysis was performed, leaving out the only study conducted in Asia. All statistical analyses were performed using Stata software version 14. A two-sided p value < 0.05 was considered statistically significant.
Results
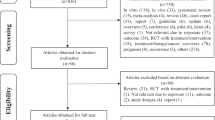
Of the nineteen case–control studies, six studies were excluded for providing no data on fruit or vegetable consumption, and two studies were excluded for providing no data on portion sizes. The eleven included case–control studies [8,9,10,11,12,13,14,15,16,17,18] originated from Belgium, Italy, Sweden, China, Canada, and the USA. In total, out of 17,012 eligible participants, 871 were excluded for having missing data on fruit or vegetable intakes (n = 595), age at study entry (n = 8), or pack years (n = 268) (Fig. 1).
Baseline characteristics of the included studies are presented in Table 2. A total of 5637 BC cases and 10,504 controls were analyzed. Most of these included participants were male (68%) and Caucasian (89%). The mean age for BC was 60 years across all studies. Even though all studies made use of an FFQ to measure usual fruit and vegetable consumption (over the preceding one or two years before study enrollment), the number of fruit and vegetable items described in each questionnaire varied widely.
Total fruits
Among the eleven included studies, nine demonstrated a trend towards reduced BC risk with higher intake of total fruit, although the association was not always statistically significant (Table 3, Fig. 2). Remarkably, one study reported a non-significant increased risk (Table 3). However, upon pooled analysis, a reduction in BC risk associated with higher total fruit intake was observed. In the overall pooled analysis, greater intakes of fruit showed a reduced BC risk compared to the lowest intakes of fruit (OR 0.79; 95% CI 0.68–0.91) (Table 3). The tests for heterogeneity showed moderate heterogeneity for the association between total fruit and BC (I2 = 42.8%).
In the subgroup analysis on gender, similar results were observed in both men and women, showing a pooled decreased BC risk among those consuming the highest intakes of fruit (ORmen = 0.83; 95% CI 0.71–0.95) (Table 4), and ORwomen = 0.60; 95% CI 0.40–0.80 (Table 5)). Whilst the heterogeneity among men for the might not be considerable (I2 = 25.7%), the heterogeneity among women may represent moderate heterogeneity (I2 = 43.3%) (Appendix 2, Tables 6, 7).
Similarly, when analyzing the data according to age (i.e., 60 years and under), consistent results were observed. In the overall pooled analysis, participants younger than 60 years of age with high fruit consumption exhibited a significant decrease in BC risk (OR 0.76; 95% CI 0.64–0.88), with no considerable statistical heterogeneity observed in this group (I2 = 0.0%) (Appendix 3, Table 10). Likewise, a decreased BC risk was observed in participants 60 years or older consuming the highest intakes of fruit (OR 0.80; 95% CI 0.69–0.91), with negligible heterogeneity for this association (I2 = 5.5%) (Appendix 3, Table 11). Additionally, the sensitivity analysis, excluding the Chinese study [12], yielded similar results compared to the overall analysis (OR 0.83; 95% CI 0.72–0.93).
Citrus fruits, pome fruits, soft fruits, stone fruits and tropical fruits
No associations between high consumption of soft fruits or stone fruits and BC risk were found (Table 3). Notably, a trend indicating a lower BC risk was observed for soft fruits in four out of the five studies (Table 3), with the exception of the Stockholm case–control study, where an increased BC risk was observed. For citrus fruits, an overall decreased BC risk was noted with high consumption (OR 0.81; 95% CI 0.65–0.98). Interestingly, a similar trend of lower BC risk for citrus fruits was evident in eight out of the ten studies (Table 3), except for the Belgian case–control study on BC and the Stockholm case–control study, which reported an increased BC risk. However, the overall decreased BC risk results for citrus fruit revealed considerable heterogeneity (I2 = 66.2). Similar patterns were observed in the stratified analysis by gender and age (i.e., > 60 years and under) (Appendix 2, Tables 6, 7, Appendix 3, Tables 10, 11). Among women participants and participants aged 60 years or more, an overall decreased BC risk was observed with high consumption of citrus fruits (OR 0.56; 95% CI 0.32–0.80, and OR 0.80; 95% CI 0.62–0.99, respectively). However, both analyses showed considerable heterogeneity (I2 = 62.5%, and I2 = 53.2%, respectively). Conversely, among participants younger than 60 years, an association between the highest intakes of citrus fruits and BC risk was observed (OR 0.77; 95% CI 0.64–0.91) with no considerable statistical heterogeneity (I2 = 0.0%). In men, no association was found between greater intakes of citrus fruits and BC risk (Appendix 2, Table 6).
Overall, greater consumption of pome fruits was associated with a decreased BC risk (OR 0.76; 95% CI 0.65–0.87) and statistical heterogeneity was not considerable (I2 = 0.0%). In the subgroup analyses, pome fruit consumption (highest versus lowest intakes) was associated with a lower BC risk in men (OR 0.77, 95% CI 0.58–0.97), women (OR 0.58, 95% CI 0.42–0.73), < 60 years (OR 0.52, 95% CI 0.31–0.72), and ≥ 60 years (OR 0.85, 95% CI 0.71–0.99), with low to moderate heterogeneity (I2 = 40.2%, I2 = 0.0%, I2 = 15.3%, and I2 = 0.0, respectively) (Appendix 2, Tables 6, 7, Appendix 3, Tables 10, 11).
High consumption of tropical fruits was associated with a decreased BC risk in the overall analysis (OR 0.84; 95% CI 0.73–0.94, I2 = 20.5%). Again, it should be noted that a trend towards a decreased risk was shown in seven out of the nine studies, while the New Hampshire and Molecular epidemiology of BC studies showed a trend towards an increased BC risk. In the subgroup analyses on age, both age groups (< 60 and ≥ 60 years) showed associations between the highest intakes of tropical fruits and BC risk (OR 0.84; 95% CI 0.70–0.98 and OR 0.83; 95% CI 0.72–0.95, respectively) and no heterogeneity was observed for these associations (Appendix 3, Tables 10 and 11).
Total vegetables
Although high total vegetable consumption was associated with BC risk (OR 0.82; 95% CI 0.70–0.94), heterogeneity was observed (I2 = 52.9%) (Table 4, Fig. 3). In men, the highest intakes of total vegetables were associated with a decreased BC risk with no considerable heterogeneity (OR 0.80; 95% CI 0.71–0.88, I2 = 1.0%) (Appendix 2, Table 8). Similar results were found for participants ≥ 60 years (OR 0.81; 95% CI 0.71–0.91, I2 = 0.0%) (Appendix 3, Table 13). Greater intakes of total vegetables among participants < 60 years were significantly associated with a decreased BC risk (OR 0.70; 95% CI 0.52–0.88). However, substantial heterogeneity was observed for this association (I2 = 47.8%) (Appendix 2, Table 3). The sensitivity analysis did not change the result (OR 0.82; 95% CI 0.69–0.95).
Leaf vegetables, brassica, stalk vegetables, shoot vegetables, and tubers
No associations between high consumptions of brassica, stalk vegetables, and tubers, and BC risk were observed (Table 4). Although subject to substantial heterogeneity, associations were found for the overall high intake of leaf vegetables and decreased BC risk (OR 0.82; 95% CI 0.68–0.96). This trend was observed for nine out of the eleven studies, while the South and East China ca–co study and NESCC study showed a trend towards an increased BC risk (Table 4).
High intakes of shoot vegetables significantly decreased the BC risk with no observed substantial heterogeneity (OR 0.87; 95% 0.78–0.96, I2 = 0.0%) (Table 4). In the subgroup analyses, significant associations for leaf vegetables (OR 0.70; 95% CI 0.56–0.84) and shoot vegetables (OR 0.83; 95% CI 0.68–0.97) were found in participants < 60 years with no substantial heterogeneity (I2 = 20.0%, and I2 = 0.0%, respectively) (Appendix 3, Table 12). Although associations were found for leaf vegetables and BC risk in men, women, and participants ≥ 60 years, substantial heterogeneity was observed for these associations (Appendix 2, Tables 8, 9, Appendix 3, Table 13).
Discussion
In this large pooled analysis of eleven case–control studies, comprising 5637 cases and 10,504 controls, significant inverse associations were found between high fruit and vegetable consumption and BC risk. There were inverse associations between total fruit, citrus fruit, pome fruit, and tropical fruit consumption and BC risk. No associations were found for high consumption of soft fruits or stone fruits. For vegetable consumption, inverse associations were found between total vegetable, leaf vegetable, and shoot vegetable consumption and a BC risk. Brassica, stalk vegetables, and tubers were not associated with BC risk.
It seems plausible that substances in fruits and vegetables including minerals, phytochemicals, and antioxidant nutrients, have potentially anticarcinogenic properties to protect against the development of cancer [1, 19]. Throughout Europe and the USA, apples and pears are the most consumed pome fruits. Apples contain a wide range of phytochemicals which have been found to have strong antioxidant properties and the ability to inhibit cancer cell proliferation [20]. Hence, results from an Italian case–control study and from the EPIC study, which included data from ten European countries, confirm that greater consumption of apples and pears decreases the BC risk (OR 0.63; 95% CI 0.39–0.99, and OR 0.90; 95% CI 0.82–0.98, respectively) [21, 22]. In addition, scientific evidence suggests that vitamin C, an essential nutrient abundant in citrus fruits, exerts anticancer effects in the bladder through diverse pathways. These include a malignancy-inhibiting shift in the transcriptome, as well as elevating levels of 5-hydroxymethylcytosine [23].
Brassica vegetables also contain high levels of phytochemicals, such as glucosinolates and isothiocyanates, and are therefore expected to lower the BC risk [19, 24]. This, however, was not confirmed in this study. Leafy vegetables contain high concentrations of carotenoids that could potentially protect against the damage to DNA by scouring free radicals [25]. Hence, results of a meta-analysis indicated that per 0.2 serving increment of daily green leafy vegetable consumption, the BC risk decreases with 2% [26]. We observed evidence of an inverse association between consumption of leafy vegetables and BC risk, which is consistent with these potential biological mechanisms.
Although no previous studies investigated specifically the role of shoot vegetables or tropical fruits in BC risk, these vegetables and fruits contain a wide range of carotenoids, folates, vitamins, and carotene, which may offer protection against the development of BC [27].
In line with our research a meta-analysis conducted in East Asians showed an inverse association between total fruit intake and BC risk [23]. Furthermore, within our BLEND study investigating fruit intake and BC risk among thirteen cohort studies, an inverse association between fruit intake and BC risk was found in women, but not in men. Nor was an association found for any fruit subgroup [28]. These null associations were confirmed in a Japanese cohort study including 1,287,514 person-years of follow-up. In addition, a null association was also found for total vegetable intake and any subgroup vegetable [29]. These observed differences might be due to the difference in study design. Although both case–control and cohort studies are subject to (a different form of) selection bias, and other methodological limitations, such as measurement error, there is an important methodological difference between these different study types. While in case–control studies, the assessment of lifestyle occurs after diagnosis, in cohort studies, the assessment of lifestyle occurs prior to diagnosis. It is therefore thought that case–control studies are more prone to recall bias and provide a lower certainty of evidence than cohort studies [30, 31]. However, since some of our results have low heterogeneity, the role of bias is likely to have minimal influence on these results [32]. In addition, recall bias has been addressed and analyzed for its consequences in many epidemiological/methodological papers, and no clear answer on the magnitude of the effect of this specific type of bias could be drawn.
Several of our results revealed discrepancies among individual study findings and indicated significant heterogeneity in the effects of fruit and vegetable consumption on BC risk. This aligns with previous research highlighting the challenges of comparing diet-disease relationships globally due to variations in dietary habits and assessment methods across populations. For instance, differences in portion size estimations, nutrient databases used, and other factors contribute to result heterogeneity, complicating generalization and interpretation. Therefore, while our analysis focused on individual food items rather than overall dietary patterns, it is essential to consider the broader context of dietary diversity and assessment methods when interpreting these findings.
Strengths and limitations
This study has some limitations. First, the number of fruit and vegetable items described in each FFQ varied widely across the studies. Although it has been reported that fruit and vegetable servings increase with the number of fruit and vegetable items on a questionnaire [33], the total of fruit and vegetable questions on the FFQs did not significantly modify the association between fruit or vegetable consumption and BC risk. In addition, the number of studies included in the fruit and vegetable subgroup analyses varied depending on whether the items comprising a particular fruit or vegetable subgroup were asked on the study-specific FFQs. Consequently, the power to examine associations for some subgroups is more limited compared with that for analyses of total fruit and total vegetables. Second, while case–control studies are valuable in investigating associations, they inherently possess limitations, particularly when assessing dietary factors which may be influenced by participants' cancer status [34]. Furthermore, as mentioned previously, case–control studies are generally considered to have higher risk of recall bias than cohort studies as the selection of the controls may be subject to population stratification [30]. We acknowledge that cohort studies and randomized controlled trials generally provide stronger evidence. Despite this, our analysis accounts for potential biases by addressing low heterogeneity across studies, suggesting minimal impact on our findings [32]. At last, while limited data on other potential BC risk factors such as body mass index and socioeconomic status were available, current literature suggests their contribution to BC risk is relatively small [35,36,37,38]. Besides, this pooled analysis also has several strengths, including the large sample size with harmonized variables across multiple studies, providing high statistical power to examine the role of total fruits and vegetables, subgroups of fruits and vegetables, and the possibility to perform subgroup analyses on gender and age. Moreover, we found low heterogeneity between studies for many of the association reported, the role of bias is likely to have minimal influence on our results.
Conclusion
This comprehensive study provides compelling evidence that the consumption of fruits overall, citrus fruits, pome fruits and tropical fruits reduce the BC risk. Besides, evidence was found for an inverse association between total vegetables and shoot vegetables intake.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Liu RH (2013) Dietary bioactive compounds and their health implications. J Food Sci 78(Suppl 1):A18-25. https://doi.org/10.1111/1750-3841.12101
Clinton SK, Giovannucci EL, Hursting SD (2020) The World Cancer Research Fund/American Institute for Cancer Research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr 150(4):663–671. https://doi.org/10.1093/jn/nxz268
Al-Zalabani AH, Stewart KF, Wesselius A, Schols AM, Zeegers MP (2016) Modifiable risk factors for the prevention of bladder cancer: a systematic review of meta-analyses. Eur J Epidemiol 31(9):811–851. https://doi.org/10.1007/s10654-016-0138-6
Fankhauser CD, Mostafid H (2018) Prevention of bladder cancer incidence and recurrence: nutrition and lifestyle. Curr Opin Urol 28(1):88–92. https://doi.org/10.1097/mou.0000000000000452
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47. https://doi.org/10.1017/jns.2016.41
Goossens ME, Isa F, Brinkman M, Mak D, Reulen R, Wesselius A, Benhamou S, Bosetti C, Bueno-de-Mesquita B, Carta A, Allam MF, Golka K, Grant EJ, Jiang X, Johnson KC, Karagas MR, Kellen E, La Vecchia C, Lu CM, Marshall J, Moysich K, Pohlabeln H, Porru S, Steineck G, Stern MC, Tang L, Taylor JA, van den Brandt P, Villeneuve PJ, Wakai K, Weiderpass E, White E, Wolk A, Zhang ZF, Buntinx F, Zeegers MP (2016) International pooled study on diet and bladder cancer: the bladder cancer, epidemiology and nutritional determinants (BLEND) study: design and baseline characteristics. Arch Public Health 74:30. https://doi.org/10.1186/s13690-016-0140-1
Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, Berrino F, van den Brandt PA, Buring JE, Cho E, Colditz GA, Folsom AR, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Harnack L, Horn-Ross PL, Krogh V, Leitzmann MF, McCullough ML, Miller AB, Rodriguez C, Rohan TE, Schatzkin A, Shore R, Virtanen M, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Hunter DJ (2006) Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol 163(11):1053–1064. https://doi.org/10.1093/aje/kwj127
Augustsson K, Skog K, Jägerstad M, Dickman PW, Steineck G (1999) Dietary heterocyclic amines and cancer of the colon, rectum, bladder, and kidney: a population-based study. Lancet 353(9154):703–707. https://doi.org/10.1016/s0140-6736(98)06099-1
Cao W, Cai L, Rao JY, Pantuck A, Lu ML, Dalbagni G, Reuter V, Scher H, Cordon-Cardo C, Figlin RA, Belldegrun A, Zhang ZF (2005) Tobacco smoking, GSTP1 polymorphism, and bladder carcinoma. Cancer 104(11):2400–2408. https://doi.org/10.1002/cncr.21446
Castelao JE, Yuan JM, Gago-Dominguez M, Skipper PL, Tannenbaum SR, Chan KK, Watson MA, Bell DA, Coetzee GA, Ross RK, Yu MC (2004) Carotenoids/vitamin C and smoking-related bladder cancer. Int J Cancer 110(3):417–423. https://doi.org/10.1002/ijc.20104
Gaertner RR, Trpeski L, Johnson KC (2004) A case–control study of occupational risk factors for bladder cancer in Canada. Cancer Causes Control 15(10):1007–1019. https://doi.org/10.1007/s10552-004-1448-7
Hemelt M, Hu Z, Zhong Z, Xie LP, Wong YC, Tam PC, Cheng KK, Ye Z, Bi X, Lu Q, Mao Y, Zhong WD, Zeegers MP (2010) Fluid intake and the risk of bladder cancer: results from the South and East China case-control study on bladder cancer. Int J Cancer 127(3):638–645. https://doi.org/10.1002/ijc.25084
Karagas MR, Tosteson TD, Blum J, Morris JS, Baron JA, Klaue B (1998) Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a US population. Environ Health Perspect 106(Suppl 4):1047–1050. https://doi.org/10.1289/ehp.98106s41047
Kellen E, Zeegers M, Paulussen A, Van Dongen M, Buntinx F (2006) Fruit consumption reduces the effect of smoking on bladder cancer risk. The Belgian case control study on bladder cancer. Int J Cancer 118(10):2572–2578. https://doi.org/10.1002/ijc.21714
Polesel J, Bosetti C, di Maso M, Montella M, Libra M, Garbeglio A, Zucchetto A, Turati F, Talamini R, La Vecchia C, Serraino D (2014) Duration and intensity of tobacco smoking and the risk of papillary and non-papillary transitional cell carcinoma of the bladder. Cancer Causes Control 25(9):1151–1158. https://doi.org/10.1007/s10552-014-0416-0
Randi G, Pelucchi C, Negri E, Talamini R, Galeone C, Franceschi S, La Vecchia C (2007) Family history of urogenital cancers in patients with bladder, renal cell and prostate cancers. Int J Cancer 121(12):2748–2752. https://doi.org/10.1002/ijc.23037
Shen M, Hung RJ, Brennan P, Malaveille C, Donato F, Placidi D, Carta A, Hautefeuille A, Boffetta P, Porru S (2003) Polymorphisms of the DNA repair genes XRCC1, XRCC3, XPD, interaction with environmental exposures, and bladder cancer risk in a case–control study in northern Italy. Cancer Epidemiol Biomark Prev 12(11 Pt 1):1234–1240
Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE (2008) Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol Biomark Prev 17(4):938–944. https://doi.org/10.1158/1055-9965.epi-07-2502
Abbaoui B, Lucas CR, Riedl KM, Clinton SK, Mortazavi A (2018) Cruciferous vegetables, isothiocyanates, and bladder cancer prevention. Mol Nutr Food Res 62(18):e1800079. https://doi.org/10.1002/mnfr.201800079
Boyer J, Liu RH (2004) Apple phytochemicals and their health benefits. Nutr J 3:5. https://doi.org/10.1186/1475-2891-3-5
Büchner FL, Bueno-de-Mesquita HB, Ros MM, Kampman E, Egevad L, Overvad K, Raaschou-Nielsen O, Tjønneland A, Roswall N, Clavel-Chapelon F, Boutron-Ruault MC, Touillaud M, Chang-Claude J, Kaaks R, Boeing H, Weikert S, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Sieri S, Vineis P, Tumino R, Panico S, Vrieling A, Peeters PH, van Gils CH, Lund E, Gram IT, Engeset D, Martinez C, Gonzalez CA, Larrañaga N, Ardanaz E, Navarro C, Rodríguez L, Manjer J, Ehrnström RA, Hallmans G, Ljungberg B, Allen NE, Roddam AW, Bingham S, Khaw KT, Slimani N, Boffetta P, Jenab M, Mouw T, Michaud DS, Kiemeney LA, Riboli E (2009) Consumption of vegetables and fruit and the risk of bladder cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 125(11):2643–2651. https://doi.org/10.1002/ijc.24582
Sacerdote C, Matullo G, Polidoro S, Gamberini S, Piazza A, Karagas MR, Rolle L, De Stefanis P, Casetta G, Morabito F, Vineis P, Guarrera S (2007) Intake of fruits and vegetables and polymorphisms in DNA repair genes in bladder cancer. Mutagenesis 22(4):281–285. https://doi.org/10.1093/mutage/gem014
Xenou D, Tzelves L, Terpos E, Stamatelopoulos K, Sergentanis TN, Psaltopoulou T (2022) Consumption of fruits, vegetables and bladder cancer risk: a systematic review and meta-analysis of prospective cohort studies. Nutr Cancer 74(6):2003–2016. https://doi.org/10.1080/01635581.2021.1985146
He C, Buongiorno LP, Wang W, Tang JCY, Miceli N, Taviano MF, Shan Y, Bao Y (2021) The inhibitory effect of sulforaphane on bladder cancer cell depends on GSH depletion-induced by Nrf2 translocation. Molecules. https://doi.org/10.3390/molecules26164919
Azqueta A, Collins AR (2012) Carotenoids and DNA damage. Mutat Res 733(1–2):4–13. https://doi.org/10.1016/j.mrfmmm.2012.03.005
Xu C, Zeng XT, Liu TZ, Zhang C, Yang ZH, Li S, Chen XY (2015) Fruits and vegetables intake and risk of bladder cancer: a PRISMA-compliant systematic review and dose-response meta-analysis of prospective cohort studies. Medicine (Baltimore) 94(17):e759. https://doi.org/10.1097/md.0000000000000759
Brinkman MT, Karagas MR, Zens MS, Schned A, Reulen RC, Zeegers MP (2010) Minerals and vitamins and the risk of bladder cancer: results from the New Hampshire Study. Cancer Causes Control 21(4):609–619. https://doi.org/10.1007/s10552-009-9490-0
Jochems SHJ, Reulen RC, van Osch FHM, Witlox WJA, Goossens ME, Brinkman M, Giles GG, Milne RL, van den Brandt PA, White E, Weiderpass E, Huybrechts I, Hémon B, Agudo A, Bueno-de-Mesquita B, Cheng KK, van Schooten FJ, Bryan RT, Wesselius A, Zeegers MP (2020) Fruit consumption and the risk of bladder cancer: a pooled analysis by the Bladder Cancer Epidemiology and Nutritional Determinants Study. Int J Cancer 147(8):2091–2100. https://doi.org/10.1002/ijc.33008
Narii N, Sobue T, Zha L, Kitamura T, Sawada N, Iwasaki M, Inoue M, Yamaji T, Tsugane S (2022) Vegetable and fruit intake and the risk of bladder cancer: Japan Public Health Center-based prospective study. Br J Cancer 126(11):1647–1658. https://doi.org/10.1038/s41416-022-01739-0
Gibson TM, Ferrucci LM, Tangrea JA, Schatzkin A (2010) Epidemiological and clinical studies of nutrition. Semin Oncol 37(3):282–296. https://doi.org/10.1053/j.seminoncol.2010.05.011
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Wesselius A, Zeegers M (2017) Re: Lifestyle and bladder cancer prevention: no consistent evidence from cohort studies. Eur J Epidemiol 32(11):1037–1038. https://doi.org/10.1007/s10654-017-0307-2
Krebs-Smith SM, Heimendinger J, Subar AF, Patterson BH, Pivonka E (1995) Using food frequency questionnaires to estimate fruit and vegetable intake: association between the number of questions and total intakes. J Nutr Educ 27(2):80–85. https://doi.org/10.1016/S0022-3182(12)80346-3
Kristal AR, Peters U, Potter JD (2005) Is it time to abandon the food frequency questionnaire? Cancer Epidemiol Biomark Prev 14(12):2826–2828. https://doi.org/10.1158/1055-9965.epi-12-ed1
Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C, Shariat S, Lotan Y (2013) Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63(2):234–241. https://doi.org/10.1016/j.eururo.2012.07.033
Dugué PA, Brinkman MT, Hodge AM, Bassett JK, Bolton D, Longano A, Hopper JL, Southey MC, English DR, Milne RL, Giles GG (2018) Dietary intake of nutrients involved in one-carbon metabolism and risk of urothelial cell carcinoma: a prospective cohort study. Int J Cancer 143(2):298–306. https://doi.org/10.1002/ijc.31319
Koebnick C, Michaud D, Moore SC, Park Y, Hollenbeck A, Ballard-Barbash R, Schatzkin A, Leitzmann MF (2008) Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiol Biomark Prev 17(5):1214–1221. https://doi.org/10.1158/1055-9965.epi-08-0026
Madeb R, Messing EM (2004) Gender, racial and age differences in bladder cancer incidence and mortality. Urol Oncol 22(2):86–92. https://doi.org/10.1016/s1078-1439(03)00139-x
Jiang X, Castelao JE, Groshen S, Cortessis VK, Ross RK, Conti DV, Gago-Dominguez M (2007) Alcohol consumption and risk of bladder cancer in Los Angeles County. Int J Cancer 121(4):839–845. https://doi.org/10.1002/ijc.22743
D’Avanzo B, La Vecchia C, Negri E, Decarli A, Benichou J (1995) Attributable risks for bladder cancer in northern Italy. Ann Epidemiol 5(6):427–431. https://doi.org/10.1016/1047-2797(95)00057-7
Johnson KC, Mao Y, Argo J, Dubois S, Semenciw R, Lava J (1998) The National Enhanced Cancer Surveillance System: a case–control approach to environment-related cancer surveillance in Canada. Environmetrics 9(5):495–504. https://doi.org/10.1002/(SICI)1099-095X(199809/10)9:5%3c495::AID-ENV318%3e3.0.CO;2-H
Steineck G, Hagman U, Gerhardsson M, Norell SE (1990) Vitamin A supplements, fried foods, fat and urothelial cancer. A case-referent study in Stockholm in 1985–87. Int J Cancer 45(6):1006–1011. https://doi.org/10.1002/ijc.2910450604
Di Maso M, Bosetti C, Taborelli M, Montella M, Libra M, Zucchetto A, Turati F, Parpinel M, Negri E, Tavani A, Serraino D, Ferraroni M, La Vecchia C, Polesel J (2016) Dietary water intake and bladder cancer risk: an Italian case–control study. Cancer Epidemiol 45:151–156. https://doi.org/10.1016/j.canep.2016.09.015
Acknowledgements
We gratefully acknowledge all principal investigators for their willingness to participate in this joint project.
Funding
The Stockholm Case–control study was supported by grant from the Swedish National Cancer Society and from the Swedish Work Environment Fund. The Roswell Park Memorial Institute Case–control study on bladder cancer was supported by Public Health Service Grants CA11535 and CA16056 from the National Cancer Institute. The Italian Case–control study on bladder cancer was conducted within the framework of the CNR (Italian National Research Council) Applied Project “Clinical Application of Oncological Research” (contracts 94.01321.PF39 and 94.01119.PF39), and with the contributions of the Italian Association for Cancer Research, the Italian League against Tumours, Milan, and Mrs. Angela Marchegiano Borgomainerio. The Brescia bladder cancer study was partly supported by the International Agency for Research on Cancer. The Molecular Epidemiology of Bladder Cancer and Prostate Cancer was supported in part by grants ES06718 (to Z.-F.Z.), U01 CA96116 (to A.B.), and CA09142 from the NIH National Institute of Environmental Health Sciences, the National Cancer Institute, the Department of Health and Human Services, and by the Ann Fitzpatrick Alper Program in Environmental Genomics at the Jonsson Comprehensive Cancer Center, UCLA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflict of interest.
Ethical approval protocol numbers
The Los Angeles Bladder cancer study: HS-05-00222; South and Easst China case–control study: not yet available; Belgian case–control study: not yet available; New Hampshire bladder cancer study: not yet available; Italian case–control study on bladder cancer: INT32/09; Molecular epidemiology of bladder cancer study: IRB#11-001325; NECSS: not yet available; Brescia bladder cancer study: 2859/9185; Roswell park cancer institute: EDR 122807; Stockholm case–control study: not yet available; Italian case–control study: not yet available.
Appendices
Appendix 1: Food frequency questionnaires (Table 5)
Appendix 2: Gender stratified results (Tables 6, 7, 8, 9)
Appendix 3: Age stratified results (Tables 10, 11, 12, 13)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boot, I.W.A., Wesselius, A., Jochems, S.H.J. et al. Fruits and vegetables intake and bladder cancer risk: a pooled analysis from 11 case–control studies in the BLadder cancer Epidemiology and Nutritional Determinants (BLEND) consortium. Eur J Nutr (2024). https://doi.org/10.1007/s00394-024-03436-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00394-024-03436-5