Abstract
Purpose
Stable isotope ratios of nitrogen (δ15N) have previously been shown to increase in human hair during periods of catabolism. The goal of this study was to assess changes in δ15N in urinary urea (δ15Nurea) and Δ15N during a short-term controlled energy deficit.
Methods
We analyzed samples from 6 recreationally active men (25 ± 1 years, BMI: 23.5 ± 0.6 kg/m2) who participated in a repeated measures cross-over study involving 4 days of energy deficit (ED, ~ 15 kcal/kg FFM) without and with exercise (ED-EX, ED + EX) and control conditions in energy balance (CON-EX, CON + EX). δ15Nurea was analyzed from urine samples, and Δ15N was calculated as δ15Nurea–δ15Ndiet, with δ15Ndiet obtained from diet prescriptions.
Results
δ15Nurea was significantly elevated in ED-EX (4.4 ± 0.2‰) when compared to CON-EX (3.7 ± 0.1‰; p = 0.026) and CON + EX (3.34 ± 0.13‰, p = 0.001). As a consequence, Δ15N was positive in ED-EX (0.2 ± 0.2‰) and remained negative in ED + EX (− 0.6 ± 0.5‰), CON-EX (− 1.0 ± 0.2) and CON + EX (− 1.1 ± 0.2). Differences in Δ15N were significant between ED-EX and CON-EX (p = 0.005) and ED-EX and CON + EX (p = 0.006).
Conclusion
Our results suggest that δ15Nurea and subsequently Δ15N are responsive to a short-term energy deficit, likely due to increased amino acid oxidation to meet energy demands and preferable elimination of 14N.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Body protein represents the largest reservoir of amino acids (AAs) and nitrogen in humans. On a daily basis, there is a constant uptake and release of AAs from different tissues to the free AA pool, which ensures constant supply of AAs for protein synthesis and availability of carbon and nitrogen for other metabolic processes, such as gluconeogenesis or synthesis of neurotransmitters [1]. These metabolic fluxes are regulated by dietary factors, such as protein and energy intake and change with altering physiological demands. For example, in an energy deficit (ED), when energy requirements are met by the breakdown of body energy stores, increased proteolysis ensures supply with AAs for gluconeogenesis [2]. Sustained energy deficits are associated with a negative nitrogen balance and the loss of body protein, as approximately 25% of weight loss is accounted by fat-free mass (FFM) [3].
One biomarker, which appears to reflect periods of negative nitrogen balance, is the ratio of naturally occurring stable nitrogen isotopes (δ15N) [4, 5]. Generally, δ15N in body tissues reflects the isotope ratio of the diet, allowing for the classification of the trophic level in animals or the differentiation between plant- vs. animal-based diet in humans [6, 7]. δ15N has also been widely used to characterize dietary habits in archeological, ecological and epidemiological studies [8]. However, when compared to the diet, body tissues are typically enriched in the heavier isotope 15N, as the lighter isotope 14N is preferably metabolized and excreted due to discrimination against 15N during trans- and deamination [9].
At the same time, δ15N and Δ15N are impacted by physiological demands. Evidence shows that δ15N increases in various tissues in both animals and humans in response to inadequate dietary intake or starvation [10,11,12,13]. In humans, most studies have measured δ15N in hair (δ15Nhair), which has been shown to be consistently elevated during periods of extreme energy deficiency, such as starvation or anorexia nervosa [13,14,15]. We have previously measured δ15Nhair over the course of a 25-day expedition, during which food intake was strongly limited and substantial weight loss occurred [16]. Anecdotally, δ15Nhair was highest when the energy deficit was greatest [16, 17]. Similarly, δ15Nhair also increased in pregnant women experiencing weight loss due to morning sickness [18], and data from animal studies also show that δ15N increases in various tissues in response to prolonged energy deficiency [10,11,12, 19]. Although the exact mechanisms for the elevation in δ15N during energy deficiency remain to be elucidated, it has been proposed that the increased breakdown of body protein leads to release of 15N-enriched AAs, which are subsequently recycled [4]. However, using data from multiple tissues and nitrogen pools obtained from rats, Poupin et al. demonstrated that δ15N of tissues is modulated by several metabolic pathways [20].
Despite successful characterization of periods of catabolism in humans, δ15Nhair only allows for retrospective analysis with a resolution of days to weeks [21, 22]. Given that urea is the end-product of AA catabolism and reflective of AA oxidation, measurements of δ15N in urinary urea (δ15Nurea) might serve as a more immediate alternative which could provide greater and timely resolution. In fact, within the first few days of fasting, excretion of urinary urea increases as a consequence of increased protein degradation and subsequent use of AA as substrates for gluconeogenesis [23].
Preliminary data from our group suggest that δ15Nurea is highly sensitive to short-term changes in dietary intakes and does not require large sample sizes [21]. Yet, it has to be confirmed that δ15Nurea is also impacted during short-term ED. To assess the potential of δ15Nurea and Δ15N as indicators of catabolism, we performed a proof-of principle analysis on samples from a previously published study employing an ED with and without exercise [24]. Our primary working hypotheses was that δ15Nurea and consequently Δ15N would increase during ED, indicating increased rates of AA oxidation in the energy-deficient state.
Materials and methods
Experimental design
For the present investigation, we analyzed samples from a randomized controlled trial assessing the impact of an ED with and without exercise on metabolic and behavioral adaptations [24, 25]. To test the independent and combined effects of an ED and exercise, the study was conducted using a four-way cross-over design during which participants underwent two 4-day conditions of ED and two 4-day control conditions in energy balance (CON) (Fig. 1). During one ED and one CON condition, participants conducted aerobic exercise (ED + EX; CON + EX). During the other ED and CON conditions, no exercise was conducted (ED-EX; CON-EX). To generate isocaloric conditions within ED and CON, dietary energy intake was adjusted for the energy expended during exercise. The order of the experimental conditions was randomly assigned. After each condition, participants underwent wash out periods of ad libitum food intake. Wash-out periods were set to at least 4 days following CON and at least 10 days following ED conditions. The study was approved by the ethical review board of the German Sport University Cologne (no approval number was given) and was conducted in accordance with the Declaration of Helsinki.
Overview of the study design. In this four-way cross-over study, participants underwent 4 days of either energy deficit (ED) or energy balance (CON) without (-EX) or with exercise (+ EX). Energy deficit and energy balance were operationally defined as an energy availability of 15 kcal/kg/fat-free mass (FFM) or 40 kcal/kg/FFM, respectively
Participants
Participants were recruited from the university community in accordance with the following inclusion criteria: male, 18–30 years of age, ≥ 3 h/week aerobic exercise, normal (body-mass index: 19–25 kg/m2; body fat percentage ≤15%), and stable body weight (± 3 kg during the past 6 months). Exclusion criteria were: smoking, infectious disease within the past 4 weeks, intake of medication that could influence the study outcomes, cardiovascular disease or orthopedic impairment interfering with conducting exercise, diabetes or history of a clinical eating disorder. All participants provided written informed consent prior to participation in the study.
Body weight and body composition
At baseline and the beginning and end of each condition, body weight and body composition were assessed with a bioimpedance scale (Tanita BC 418 MA, Tanita, Amsterdam, The Netherlands). All measurements were carried out in the morning with participants in a fasted (≥ 10 h) and well-hydrated state [24].
Diet prescription
Energy deficit was operationally achieved by reducing energy availability, defined as dietary energy intake minus exercise energy expenditure, to 15 kcal/kg FFM, which represents approximately 50% of basal energy requirements [26]. Energy balance was assumed at an energy availability of 40 kcal/kg FFM [27]. Detailed meal plans were developed for each participant, based on individual dietary habits and food preferences. The meal plans served to ensure energy intake was in accordance with the respective conditions. Regarding macronutrient distribution, it was aimed to keep the intakes within the range of the recommendations of the German Nutrition Association (50–55% carbohydrates, 30–35% fat, 10–15% protein) while also assuring that protein intake at least met the recommended daily allowance for adults (0.8 g/kg) [28]. During all conditions, participants weighed all foods consumed as well as leftovers with a food scale and reported their intake daily. Analysis of food consumed occurred on a daily basis (EBIS pro version 7.0, University of Hohenheim, Stuttgart, Germany, 2005) and meal plans were adjusted if reported and prescribed intake differed by ≥ 50 kcal.
Exercise prescription
During exercise conditions, participants performed supervised exercise on the bicycle ergometer at 60% of their VO2peak until an exercise energy expenditure of 15 kcal/kg FFM was achieved. Outside of the intervention, participants were instructed to abstain from any additional exercise, which was monitored with an activity tracker (SenseWear Pro3 armband, Bodymedia, Pittsburgh, USA).
Stable nitrogen isotope ratio analysis and calculations
For analysis of δ15Nurea, participants were asked to collect three urine samples per day. Samples were collected in the morning in a fasted state as well as in the early afternoon and at bedtime. Participants were instructed to record the time of sample collection and the total urine volume. A 50-mL aliquot of each sample was stored in the participants’ refrigerators until delivery to the laboratory, which occurred within 24–48 h. For precipitation of urinary urea, 250 μL of urine were acidified with 375 μL of glacial acetic acid (analytical grade; Merck, Darmstadt, Germany) and 375 μL 10% (w/w) xanthydrol (Sigma-Aldrich, Steinheim, Germany) in methanol (analytical grade; VWR, Darmstadt, Germany) were added and stored at 6 °C for 12 h [21]. Thereafter, the mixture was centrifuged, the supernatant discarded and the precipitate (N,N′-dixanthylurea) washed twice with methanol/water (2:1 v/v). N,N′-dixanthylurea was dried overnight in vacuum over phosphorus pentoxide. For analysis, ~ 500 μg N,N′-dixanthylurea were weighed into tin capsules and δ15N of urea was assessed using elemental analysis–isotope ratio mass spectrometry. Isotope ratios are expressed relative to atmospheric nitrogen (AIR). The elemental analyzer (Eurovektor EA 3000, Hekatech, Wegberg, Germany) was coupled to a Delta C continuous-flow isotope ratio mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). The working standard gas (N2, purity 5.0, Linde, Munich, Germany) and the working standard (creatine-monohydrate, AlzChem, Trostberg, Germany) were scale calibrated using IAEA-N-1 (+ 0.4 ‰) and IAEA-N-2 (+ 20.3 ‰) for δ15N values (IAEA, Vienna, Austria). All measurements were carried out in triplicates and the standard deviation for triplicate measure of the working standard was ± 0.2‰. Instrument stability and analytical performance were checked by regular analysis of the working standard and zero blanks. For the present analysis, data from the three daily samples were aggregated into a daily average of δ15Nurea, and δ15Nurea was further averaged across each of the 4-day study condition. Δ15N was calculated as the difference between δ15Nurea and dietary nitrogen isotope ratio (δ15Ndiet), which was obtained from the prescribed diet as previously described [29].
Statistical analysis
Statistical analyses were performed with R (version 4.1.1). If not stated otherwise, data are reported as mean ± standard error of the mean (SEM). To account for the small sample size and missing data from one participant, we used linear models to identify changes within conditions, between conditions, and over time. The subject identifier was included in all models to account for the repeated measures. If a trend was observed (p < 0.1), paired one sided t-tests were performed as post-hoc analyses and p-values were adjusted for multiple testing using Holm-correction. Statistical significance was set at < 0.05. Effect sizes were calculated from the difference of means and standard deviation of those differences, with d = 0.2 considered as small, d = 0.5 as medium and d > 0.8 as large effect [30]. To evaluate the impact of potential confounders on Δ15N, multiple linear regression analysis was performed using Δ15N as dependent and protein intake (in g/day), energy availability (in kcal/kg FFM) and the interaction as independent variables. Subject identifiers were included in all models and adjusted R2 was interpreted. The standardized coefficient (β) was obtained from multiple linear regression with Z-transformed variables. Sample size was predetermined through power calculations performed for the primary analysis [24].
Results
Study participants
Participants were 25 ± 1 years old, had a BMI of 23.5 ± 0.6 kg/m2, and physically trained according to their VO2peak (49.3 ± 2.4 ml/kg/min). Participants completed all conditions, adherence to the prescribed diet was high (97–106% of the prescribed energy intake), and attended all supervised exercise sessions (Table 1). Protein intake was significantly lower in ED-EX when compared to ED + EX,CON-EX and CON + EX (all p < 0.01).
Changes in body weight and composition
Significant reductions in body weight occurred in both ED conditions (Table 2). In ED-EX, losses were primarily attributed to reductions in FFM (67%), whereas in ED + EX, fat mass and FFM losses contributed almost equally.
Stable nitrogen isotope ratio analysis
Because one participant failed to provide urine samples on days 1–3 of the ED + EX condition, δ15Nurea and Δ15N data are from n = 5 for this condition. For all other conditions, data is available for all 6 participants. As shown in Fig. 2, δ15Nurea generally followed δ15Ndiet. δ15Nurea increased over the course of CON-EX (p < 0.001) but not in any other condition. There was no significant time trend observed for Δ15N. We therefore aggregated isotope data across all intervention days for further analyses.
Changes in the 15N/14N isotope ratios (mean ± SEM) in urinary urea (δ15Nurea) over the course of each intervention. Gray shaded areas denote the range of 15N/14N isotope ratios in the diet (δ15Ndiet). ED-EX energy deficit without exercise; ED + EX energy deficit with exercise; CON-EX control without exercise; CON + EX control with exercise
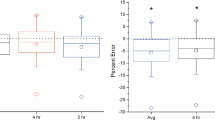
Mean δ15Nurea was significantly higher in ED-EX (4.4 ± 0.2‰) when compared to CON-EX (3.7 ± 0.1‰; p = 0.026, d = 1.63) and CON + EX (3.3 ± 0.1‰, p = 0.001, d = 3.52). Mean δ15Nurea was also higher in ED + EX when compared to CON + EX (3.8 ± 0.1‰ vs. 3.3 ± 0.1‰; p = 0.050, d = 1.51). The difference in mean δ15Nurea between ED-EX and ED + EX (4.4 ± 0.2‰ vs. 3.8 ± 0.1‰; p = 0.050, d = 1.56) also trended toward significance (Fig. 3A). There were no significant differences in δ15Ndiet between conditions (Fig. 3B). Consequently, Δ15N was positive in ED-EX (0.2 ± 0.2‰) but remained negative in ED + EX (− 0.6 ± 0.5‰) as well as both control conditions (CON-EX: − 1.0 ± 0.2‰; CON + EX: − 1.1 ± 0.2‰). When compared between conditions, Δ15N was significantly greater in ED-EX than in CON-EX (p = 0.005, d = 2.55) and CON + EX (p = 0.006, d = 2.31). Differences in Δ15N between ED + EX and CON + EX (p = 0.244) and between ED-EX and ED + EX (p = 0.244) were not significant (Fig. 3C).
Individual 15N/14N isotope ratios in urinary urea (δ15Nurea; A) and in the diet (δ15Ndiet; B) and the difference between urea and dietary δ15N (Δ15N; C) during 4 days of energy deficit without (ED-EX) and with exercise (ED + EX) and energy balance without (CON-EX) and with exercise (CON + EX). Horizontal lines represent respective group means. Labeled bars without a common letter differ, (p < 0.05)
Multiple linear regression analysis
Multiple linear regression analyses showed that ED as the primary intervention explained 39% of variation in Δ15N (p = 0.026). Exercise as the secondary intervention did not meaningfully contribute to Δ15N and was subsequently excluded from further analysis. Dietary protein intake improved the prediction of Δ15N to 64% (p = 0.001), and the interaction between ED and dietary protein intake further improved the model (Radjusted = 0.72, p < 0.001, Table 3). Altogether, the regression model suggests that increasing energy intake by 1 unit (1 kcal/kg FFM) reduces Δ15N by on average 0.076‰ and that each increase in dietary protein intake by 1 unit (1 g/day) translates into a reduction in Δ15N by on average 0.026‰. Consequentially, decreasing energy intake or protein intake both lead to an increase in Δ15N.
Discussion
The present study is the first prospective analysis of δ15Nurea and Δ15N (calculated as the difference between δ15Nurea and δ15Ndiet) as markers of a catabolic state during controlled short-term ED in humans. As hypothesized, both δ15Nurea and Δ15N increased in response to ED, which is in line with previous findings of increased δ15Nurea and Δ15N in urine in various tissues during periods of inadequate energy intake in both animal and human studies [10,11,12, 14, 18, 19]. Previous studies conducted in humans are based on retrospective analysis of δ15N measured in hair [14, 18]. Although sampling of hair represents a non-invasive and easy sampling method, hair has a resolution of only days to weeks. In contrast, measurement of δ15N in urinary urea as the main end-product of amino acid catabolism allows greater resolution [21].
It is well established, that δ15N values of urine are generally lower when compared to other body tissues [31], which is explained by preferantion of 14N during trans- and deamination [9, 32]. In line, when compared to δ15N measured in hair, δ15Nurea appears to be lower and closer to the diet [21]. In a previous validation study, we reported δ15Nurea values within the range of 3.5–5.4 ‰ with a mean of 4.8 ‰, which were closely associated with δ15Ndiet (4.1–5.4 ‰, mean: 4.6 ‰). In the present study, we determined a comparable range in δ15Ndiet (3.7–5.9 ‰), with similar means within each condition. Although in the same overall range, δ15Nurea mean values were higher than δ15Ndiet in ED-EX (4.4 ± 0.2‰ vs. 4.1 ± 0.2‰) and on average only about 0.7‰ below δ15Ndiet in ED + EX (3.8 ± 0.1‰ vs. 4.5 ± 0.4‰). In contrast, the gap between δ15Nurea and δ15Ndiet was almost 1‰ in CON-EX (3.7 ± 0.1‰ vs. 4.6 ± 0.2‰) and CON + EX (3.3 ± 0.1‰ vs. 4.4 ± 0.2‰). Thereby, we demonstrate that a short-term energy deficit is associated with increased δ15Nurea values, which are closer to or even exceed δ15Ndiet, resulting in an increased (“less negative” or even positive) Δ15N. These findings are also in line with previous results from animal studies reporting increases in δ15N in urine or urinary urea during periods of inadequate energy (and protein) intake [11, 19, 33, 34]. It has been suggested that changes in δ15N could be indicative of negative nitrogen balance and increased loss of body protein [5]. Indeed, results from animal studies confirm this by showing a positive relationship between losses of lean mass and increasing δ15N values in urine [34]. In humans, studies analyzing δ15N in hair of anorexia nervosa patients showed elevated values which decreased again during rehabilitation [13]. Similarly, weight loss due to morning sickness in pregnant women was also traceable as increased δ15N in hair samples [18]. Although our experiment was not suited to establish a direct relationship between loss of body protein and δ15Nurea, we observed a negative trend between δ15Nurea and changes in body weight (r = − 0.62, data not shown). In addition, due to available data on δ15Ndiet, we were also able to calculate Δ15N, which was not possible in previously mentioned human studies.
Overall, it appears that isotopic fractionation is complex and δ15N as well as Δ15N not only vary between tissues [35], but also change in response to an energy deficit. In fact, in a tightly controlled animal study employing controlled calorie restriction in rats, Huneau et al. demonstrated in vivo, that changes in isotopic signatures can be viewed as “fingerprints” of AA flux directed toward oxidation or protein synthesis. In their study, the authors report increases in Δ15N in urine and tissues such as the liver, with a concomitant decrease in Δ15N in skeletal muscle. Based on a previously published model [20], the authors suggested that these contrasting findings are due to maintained, preferable elimination of 14N AAs in tissues such as the liver, while the muscle, which represents the largest reservoir of AAs, releases proportionally more 15N-enriched AAs, resulting in a decrease in Δ15N. Notably, in the above mentioned study, the increase in Δ15N in urine was associated with a loss of liver protein mass [19]. Although we cannot confirm the role of protein losses from tissues to increases in δ15Nurea and Δ15N, respectively, we explain the increase by means of increased oxidation of AAs and preferable elimination of 14N in the liver, which supports increased demand for substrates for gluconeogenesis during an energy and carbohydrate deficit [20]. The preferable elimination of 14N AAs would also fall in line with the “anabolic model” described by Lee et al. This model postulates that during periods of catabolism, the excretion of 14N results in the release of proportionally more 15N AAs into the free AA pool, which are subsequently used for tissue protein synthesis [34]. Yet, the current study does not allow us to draw conclusions about δ15N of tissues. Considering that our study period was only 4 days, we acknowledge that participants were neither in an isotopic nor an elemental steady state condition. For the analysis of δ15Nurea and Δ15N as prospective markers with timely resolution, a steady state was not desirable.
However, we can be certain that the participants were all in negative energy balance, which we could verify indirectly through the noticeable weight loss in both ED conditions. While weight loss was similar in the two ED conditions, the composition of weight loss was impacted by exercise. While 67% of weight loss during the ED-EX condition was attributed to loss of FFM, only ~ 50% were attributed to FFM in ED + EX. The difference of approximately 17% falls in line with estimations from Weinheimer et al. [3]. Given the protective effect of exercise on FFM, it is speculated that exercise could attenuate the decrease in Δ15N in skeletal muscle, as a consequence of reduced loss of skeletal muscle mass.
In addition to exercise, protein intake is an important modulator of AA metabolism and high-protein diets are commonly prescribed to maximize retention of FFM during weight loss. While energy intake was tightly controlled, we did not control for protein quantity, resulting in comparable intakes between the CON and ED + EX condition, but a lower intake in ED-EX. Although we acknowledge the lack of standardized protein intake as limitation, we accounted for quality of the diet and estimated δ15Ndiet which allowed calculation of Δ15N. The importance of taking δ15Ndiet into account is necessary, as we already reported differing δ15Nurea values in response to different protein sources such as meat or seafood [21].
Finally, we acknowledge that our analysis had a small sample size and was exploratory in nature. Hence, further tightly controlled studies are needed to evaluate the magnitudes of changes, which could be used as clinically relevant changes in δ15Nurea and Δ15N, respectively. In a clinical setting, such biomarkers could be useful in the monitoring of patients at risk for cachexia or other scenarios of rapid weight loss. As previously conducted with δ15N from hair, δ15Nurea and Δ15N could also be used to monitor patients with anorexia.
Conclusion
Our findings show that δ15Nurea and Δ15N increase during a period of catabolism and are indicative of the metabolic state. We are the first to report a measurable increase in δ15Nurea and Δ15N after as little as 4 days of ED. We propose that the increase is the result of increased AA oxidation and preferable elimination of 14N, likely in the liver. The potential of δ15N and Δ15N as prospective markers of catabolism need to be further evaluated in studies with fixed protein intake. In addition, the impact of exercise needs further clarification.
Data availability
The raw data of this article will be made available by the authors, upon reasonable request.
References
Lund P, Williamson DH (1985) Inter-tissue nitrogen fluxes. Br Med Bull 41:251–256. https://doi.org/10.1093/oxfordjournals.bmb.a072059
Pasiakos SM, Margolis LM, Orr JS (2015) Optimized dietary strategies to protect skeletal muscle mass during periods of unavoidable energy deficit. FASEB J 29:1136–1142. https://doi.org/10.1096/fj.14-266890
Weinheimer EM, Sands LP, Campbell WW (2010) A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev 68:375–388. https://doi.org/10.1111/j.1753-4887.2010.00298.x
Reitsema LJ (2013) Beyond diet reconstruction: Stable isotope applications to human physiology, health, and nutrition: Stable Isotopes and Physiology. Am J Hum Biol 25:445–456. https://doi.org/10.1002/ajhb.22398
Gannes LZ, del Rio CM, Koch P (1998) Natural abundance variations in stable isotopes and their potential uses in animal physiological ecology. Comp Biochem Physiol A: Mol Integr Physiol 119:725–737. https://doi.org/10.1016/S1095-6433(98)01016-2
Dierkes J, Dietrich S, Abraham K et al (2023) Stable isotope ratios of nitrogen and carbon as biomarkers of a vegan diet. Eur J Nutr 62:433–441. https://doi.org/10.1007/s00394-022-02992-y
O’Connell TC, Hedges REM (1999) Investigations into the effect of diet on modern human hair isotopic values. Am J Phys Anthropol 108:409–425. https://doi.org/10.1002/(SICI)1096-8644(199904)108:4%3c409::AID-AJPA3%3e3.0.CO;2-E
O’Brien DM (2015) Stable isotope ratios as biomarkers of diet for health research. Annu Rev Nutr 35:565–594. https://doi.org/10.1146/annurev-nutr-071714-034511
Schoeller DA (1999) Isotope fractionation: why aren’t we what we eat? J Archaeol Sci 26:667–673. https://doi.org/10.1006/jasc.1998.0391
Barboza PS, Shively RD, Gustine DD, Addison JA (2020) Winter is coming: conserving body protein in female reindeer, caribou, and muskoxen. Front Ecol Evol 8:150. https://doi.org/10.3389/fevo.2020.00150
Deschner T, Fuller BT, Oelze VM et al (2012) Identification of energy consumption and nutritional stress by isotopic and elemental analysis of urine in bonobos (Pan paniscus ): Isotopic analysis of nutritional stress in bonobo urine. Rapid Commun Mass Spectrom 26:69–77. https://doi.org/10.1002/rcm.5312
Gustine DD, Barboza PS, Adams LG, Wolf NB (2014) Environmental and physiological influences to isotopic ratios of n and protein status in a montane ungulate in winter. PLoS ONE 9:e103471. https://doi.org/10.1371/journal.pone.0103471
Mekota A-M, Grupe G, Ufer S, Cuntz U (2006) Serial analysis of stable nitrogen and carbon isotopes in hair: monitoring starvation and recovery phases of patients suffering from anorexia nervosa. Rapid Commun Mass Spectrom 20:1604–1610. https://doi.org/10.1002/rcm.2477
Mekota A-M, Grupe G, Ufer S, Cuntz U (2009) Identifying starvation episodes using stable isotopes in hair: forensic approach on serial hair analysis. Rechtsmedizin 19:431–440. https://doi.org/10.1007/s00194-009-0630-3
Neuberger FM, Jopp E, Graw M et al (2013) Signs of malnutrition and starvation—reconstruction of nutritional life histories by serial isotopic analyses of hair. Forensic Sci Int 226:22–32. https://doi.org/10.1016/j.forsciint.2012.10.037
Koehler K, Huelsemann F, de Marees M et al (2011) Case study: simulated and real-life energy expenditure during a 3-week expedition. Int J Sport Nutr Exerc Metab 21:520–526. https://doi.org/10.1123/ijsnem.21.6.520
Huelsemann F, Koehler K, Flenker U (2016) Effects of heavy exercise and restricted diet regimes on nitrogen balance and body composition. In: Lee-Thorp J, Katzenberg MA (eds) The Oxford Handbook of the Archaeology of Diet. Oxford University Press
Fuller BT, Fuller JL, Sage NE et al (2005) Nitrogen balance andδ15N: why you’re not what you eat during nutritional stress. Rapid Commun Mass Spectrom 19:2497–2506. https://doi.org/10.1002/rcm.2090
Huneau J-F, Mantha OL, Hermier D et al (2019) Natural isotope abundances of carbon and nitrogen in tissue proteins and amino acids as biomarkers of the decreased carbohydrate oxidation and increased amino acid oxidation induced by caloric restriction under a maintained protein intake in obese rats. Nutrients 11:1087. https://doi.org/10.3390/nu11051087
Poupin N, Mariotti F, Huneau J-F et al (2014) Natural isotopic signatures of variations in body nitrogen fluxes: a compartmental model analysis. PLoS Comput Biol 10:e1003865. https://doi.org/10.1371/journal.pcbi.1003865
Hülsemann F, Koehler K, Flenker U, Schänzer W (2017) Do we excrete what we eat? Analysis of stable nitrogen isotope ratios of human urinary urea: Nitrogen isotope ratios of urinary urea. Rapid Commun Mass Spectrom 31:1221–1227. https://doi.org/10.1002/rcm.7891
Petzke KJ, Lemke S (2009) Hair protein and amino acid 13 C and 15 N abundances take more than 4 weeks to clearly prove influences of animal protein intake in young women with a habitual daily protein consumption of more than 1 g per kg body weight. Rapid Commun Mass Spectrom 23:2411–2420. https://doi.org/10.1002/rcm.4025
Giesecke K, Magnusson I, Ahlberg M et al (1989) Protein and amino acid metabolism during early starvation as reflected by excretion of urea and methylhistidines. Metabolism 38:1196–1200. https://doi.org/10.1016/0026-0495(89)90159-5
Koehler K, Hoerner NR, Gibbs JC et al (2016) Low energy availability in exercising men is associated with reduced leptin and insulin but not with changes in other metabolic hormones. J Sports Sci 34:1921–1929. https://doi.org/10.1080/02640414.2016.1142109
Martin A, Hofmann H, Drenowatz C et al (2021) The impact of low energy availability on nonexercise activity thermogenesis and physical activity behavior in recreationally trained adults. Int J Sport Nutr Exerc Metab 31:329–336. https://doi.org/10.1123/ijsnem.2021-0029
Burke LM, Lundy B, Fahrenholtz IL, Melin AK (2018) Pitfalls of conducting and interpreting estimates of energy availability in free-living athletes. Int J Sport Nutr Exerc Metab 28:350–363. https://doi.org/10.1123/ijsnem.2018-0142
Loucks AB, Thuma JR (2003) Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab 88:297–311. https://doi.org/10.1210/jc.2002-020369
Deutsche Gesellschaft für Ernährung (2012) Referenzwerte für die Nährstoffzufuhr. Neuer Umschau Buchverlag, Neustadt an der Weinstraße
Huelsemann F, Koehler K, Braun H et al (2013) Human dietary δ 15 N intake: representative data for principle food items: representative δ 15 N values of human diet. Am J Phys Anthropol 152:58–66. https://doi.org/10.1002/ajpa.22328
Cohen J (1992) Statistical power analysis. Curr Dir Psychol Sci 1:98–101. https://doi.org/10.1111/1467-8721.ep10768783
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140. https://doi.org/10.1016/0016-7037(84)90204-7
Macko SA, Estep MLF, Engel MH, Hare PE (1986) Kinetic fractionation of stable nitrogen isotopes during amino acid transamination. Geochim Cosmochim Acta 50:2143–2146. https://doi.org/10.1016/0016-7037(86)90068-2
Barboza PS, Parker KL (2006) Body protein stores and isotopic indicators of N balance in female Reindeer ( Rangifer tarandus ) during winter. Physiol Biochem Zool 79:628–644. https://doi.org/10.1086/502811
Lee TN, Buck CL, Barnes BM, O’Brien DM (2012) A test of alternate models for increased tissue nitrogen isotope ratios during fasting in hibernating arctic ground squirrels. J Exp Biol. https://doi.org/10.1242/jeb.068528
Deniro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351. https://doi.org/10.1016/0016-7037(81)90244-1
Acknowledgements
The authors would like to thank all participants who took part in the study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded internally.
Author information
Authors and Affiliations
Contributions
PW: Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. FH: Conceptualization, Methodology, Investigation, Data curation, Writing – review & editing. KK: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Resources, Writing – review & editing, Project administration, Resources, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to disclose.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wasserfurth, P., Huelsemann, F. & Koehler, K. Changes in urinary stable nitrogen isotope ratios during controlled short-term energy deficit: a proof-of-principle analysis. Eur J Nutr 63, 919–926 (2024). https://doi.org/10.1007/s00394-023-03320-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03320-8