Abstract
Purpose
Coffee intake and apolipoprotein B levels have been linked to gastric, colorectal, and esophageal cancers in numerous recent studies. However, whether these associations are all causal remains unestablished. This study aimed to assess the potential causal associations of apolipoprotein B and coffee intake with the risk of gastric, colorectal, and esophageal cancers using Mendelian randomization analysis.
Methods
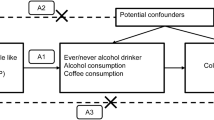
In this study, we utilized a two-sample Mendelian randomization analysis to access the causal effects of coffee intake and apolipoprotein B on gastric, colorectal, and esophageal cancers. The summary statistics of coffee intake (n = 428,860) and apolipoprotein B (n = 439,214) were obtained from the UK Biobank. In addition, the summary statistics of gastric cancer, colorectal cancer, and esophageal cancer were obtained from the FinnGen biobank (n = 218,792). Inverse variance weighted, MR–Egger, weighted median, and weighted mode were applied to examine the causal relationship between coffee intake, apolipoprotein B and gastric, colorectal, and esophageal cancers. MR–Egger intercept test, Cochran’s Q test, and leave-one-out analysis were performed to evaluate possible heterogeneity and pleiotropy. Steiger filtering and bidirectional mendelian randomization analysis were performed to evaluate the possible reverse causality.
Results
The result of the inverse variance weighted method indicated that apolipoprotein B levels were significantly associated with a higher risk of gastric cancer (OR = 1.392, 95% CI 1.027–1.889, P = 0.0333) and colorectal cancer (OR = 1.188, 95% CI 1.001–1.411, P = 0.0491). Furthermore, multivariable Mendelian randomization analysis also revealed a positive association between apolipoprotein B levels and colorectal cancer risk, but the effect of apolipoprotein B on gastric cancer risk disappeared after adjustment of coffee intake, body mass index or lipid-related traits. However, we did not discover any conclusive evidence linking coffee intake to gastric, colorectal, or esophageal cancers.
Conclusions
This study suggested a causal association between genetically increased apolipoprotein B levels and higher risk of colorectal cancer. No causal relationship was observed between coffee intake and gastric, colorectal, or esophageal cancers.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA J Clinic. https://doi.org/10.3322/caac.21590
Fernandes E, Sores J, Cotton S et al (2020) Esophageal, gastric and colorectal cancers: looking beyond classical serological biomarkers towards glycoproteomics-assisted precision oncology. Theranostics 10(11):4903–4928. https://doi.org/10.7150/thno.42480
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Morgan E, Arnold M, Gini A et al (2023) Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72(2):338–344. https://doi.org/10.1136/gutjnl-2022-327736
Hu GL, Wang X, Zhang L, Qiu MH (2019) The sources and mechanisms of bioactive ingredients in coffee. Food Funct 10(6):3113–3126. https://doi.org/10.1039/c9fo00288j
Schmit SL, Rennert HS, Rennert G, Gruber SB (2016) Coffee consumption and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 25(4):634–639. https://doi.org/10.1158/1055-9965.EPI-15-0924
Mirvish SS (1995) Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett 93(1):17–48
Dik VK, Bueno-de-Mesquita HBA, Van Oijen MGH et al (2014) Coffee and tea consumption, genotype-based CYP1A2 and NAT2 activity and colorectal cancer risk-results from the EPIC cohort study. Int J Cancer 135(2):401–412. https://doi.org/10.1002/ijc.28655
Martimianaki G, Bertuccio P, Alicandro G et al (2022) Coffee consumption and gastric cancer: a pooled analysis from the Stomach cancer Pooling Project consortium. Eur J Cancer Prev 31(2):117–127. https://doi.org/10.1097/CEJ.0000000000000680
Kim SY, Yoo DM, Min C, Choi HG (2021) Association between coffee consumption/physical exercise and gastric, hepatic, colon, breast, uterine cervix, lung, thyroid, prostate, and bladder cancer. Nutrients. https://doi.org/10.3390/nu13113927
Song H, Shen X, Chu Q, Zheng X (2022) Coffee consumption is not associated with the risk of gastric cancer: an updated systematic review and meta-analysis of prospective cohort studies. Nutr Res 102:35–44. https://doi.org/10.1016/j.nutres.2022.03.002
Zheng J-S, Yang J, Fu Y-Q, Huang T, Huang Y-J, Li D (2013) Effects of green tea, black tea, and coffee consumption on the risk of esophageal cancer: a systematic review and meta-analysis of observational studies. Nutr Cancer. https://doi.org/10.1080/01635581.2013.741762
Zamora-Ros R, Luján-Barroso L, Bueno-de-Mesquita HB et al (2014) Tea and coffee consumption and risk of esophageal cancer: the European prospective investigation into cancer and nutrition study. Int J Cancer 135(6):1470–1479. https://doi.org/10.1002/ijc.28789
Vieira AR, Abar L, Chan DSM et al (2017) Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann Oncol 28(8):1788–1802. https://doi.org/10.1093/annonc/mdx171
Zhang J, Zhou B, Hao C (2018) Coffee consumption and risk of esophageal cancer incidence: a meta-analysis of epidemiologic studies. Medicine 97(17):e0514. https://doi.org/10.1097/MD.0000000000010514
Li G, Ma D, Zhang Y, Zheng W, Wang P (2013) Coffee consumption and risk of colorectal cancer: a meta-analysis of observational studies. Public Health Nutr 16(2):346–357. https://doi.org/10.1017/S1368980012002601
Shen Z, Liu H, Cao H (2015) Coffee consumption and risk of gastric cancer: an updated meta-analysis. Clin Res Hepatol Gastroenterol 39(2):245–253. https://doi.org/10.1016/j.clinre.2014.09.005
Yu X, Bao Z, Zou J, Dong J (2011) Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer 11:96. https://doi.org/10.1186/1471-2407-11-96
Trompet S, Packard CJ, Jukema JW (2018) Plasma apolipoprotein-B is an important risk factor for cardiovascular disease, and its assessment should be routine clinical practice. Curr Opin Lipidol 29(1):51–52. https://doi.org/10.1097/MOL.0000000000000476
Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E (2001) High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet (London, England) 358(9298):2026–2033
Borgquist S, Butt T, Almgren P et al (2016) Apolipoproteins, lipids and risk of cancer. Int J Cancer 138(11):2648–2656. https://doi.org/10.1002/ijc.30013
Fang Z, He M, Song M (2021) Serum lipid profiles and risk of colorectal cancer: a prospective cohort study in the UK Biobank. Br J Cancer 124(3):663–670. https://doi.org/10.1038/s41416-020-01143-6
Ma M-Z, Yuan S-Q, Chen Y-M, Zhou Z-W (2018) Preoperative apolipoprotein B/apolipoprotein A1 ratio: a novel prognostic factor for gastric cancer. Onco Targets Ther 11:2169–2176. https://doi.org/10.2147/OTT.S156690
Pih GY, Gong EJ, Choi JY et al (2021) Associations of serum lipid level with gastric cancer risk, pathology, and prognosis. Cancer Res Treat 53(2):445–456. https://doi.org/10.4143/crt.2020.599
Faraj M, Messier L, Bastard JP et al (2006) Apolipoprotein B: a predictor of inflammatory status in postmenopausal overweight and obese women. Diabetologia 49(7):1637–1646
Foran E, Garrity-Park MM, Mureau C et al (2010) Upregulation of DNA methyltransferase-mediated gene silencing, anchorage-independent growth, and migration of colon cancer cells by interleukin-6. Mol Cancer Res 8(4):471–481. https://doi.org/10.1158/1541-7786.MCR-09-0496
Lin M-T, Lin B-R, Chang C-C et al (2007) IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int J Cancer 120(12):2600–2608
Du R, Wu X, Peng K et al (2019) Serum apolipoprotein B is associated with increased risk of metabolic syndrome among middle-aged and elderly Chinese: a cross-sectional and prospective cohort study. J Diabetes 11(9):752–760. https://doi.org/10.1111/1753-0407.12904
Castro JP, Grune T, Speckmann B (2016) The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol Chem 397(8):709–724. https://doi.org/10.1515/hsz-2015-0305
Zhou A, Hyppönen E (2021) Habitual coffee intake and plasma lipid profile: evidence from UK Biobank. Clin Nutr 40(6):4404–4413. https://doi.org/10.1016/j.clnu.2020.12.042
Williams PT, Wood PD, Vranizan KM, Albers JJ, Garay SC, Taylor CB (1985) Coffee intake and elevated cholesterol and apolipoprotein B levels in men. JAMA 253(10):1407–1411
Aro A, Teirilä J, Gref CG (1990) Dose-dependent effect on serum cholesterol and apoprotein B concentrations by consumption of boiled, non-filtered coffee. Atherosclerosis 83(2–3):257–261
Superko HR, Bortz W, Williams PT, Albers JJ, Wood PD (1991) Caffeinated and decaffeinated coffee effects on plasma lipoprotein cholesterol, apolipoproteins, and lipase activity: a controlled, randomized trial. Am J Clin Nutr 54(3):599–605
Yang J, Wei H, Zhou Y et al (2022) High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. https://doi.org/10.1053/j.gastro.2021.08.041
Lu L, Mullins CS, Schafmayer C, Zeißig S, Linnebacher M (2021) A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun (Lond) 41(11):1137–1151. https://doi.org/10.1002/cac2.12220
Davey Smith G, Hemani G (2014) Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 23(R1):R89–R98. https://doi.org/10.1093/hmg/ddu328
Davies NM, Holmes MV, Davey SG (2018) Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362:k601. https://doi.org/10.1136/bmj.k601
Li P, Wang H, Guo L et al (2022) Association between gut microbiota and preeclampsia-eclampsia: a two-sample Mendelian randomization study. BMC Med 20(1):443. https://doi.org/10.1186/s12916-022-02657-x
Emdin CA, Khera AV, Kathiresan S (2017) Mendelian randomization. JAMA 318(19):1925–1926. https://doi.org/10.1001/jama.2017.17219
Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey SG (2008) Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27(8):1133–1163
Boef AGC, Dekkers OM, le Cessie S (2015) Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol 44(2):496–511. https://doi.org/10.1093/ije/dyv071
Didelez V, Sheehan N (2007) Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res 16(4):309–330
Richardson TG, Sanderson E, Palmer TM et al (2020) Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable Mendelian randomisation analysis. PLoS Med 17(3):e1003062. https://doi.org/10.1371/journal.pmed.1003062
Zhu G-L, Xu C, Yang K-B et al (2022) Causal relationship between genetically predicted depression and cancer risk: a two-sample bi-directional Mendelian randomization. BMC Cancer 22(1):353. https://doi.org/10.1186/s12885-022-09457-9
Kamat MA, Blackshaw JA, Young R et al (2019) PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics 35(22):4851–4853. https://doi.org/10.1093/bioinformatics/btz469
Bai X, Wei H, Liu W et al (2022) Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut 71(12):2439–2450. https://doi.org/10.1136/gutjnl-2021-325021
Bagnardi V, Rota M, Botteri E et al (2015) Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 112(3):580–593. https://doi.org/10.1038/bjc.2014.579
Bardou M, Barkun AN, Martel M (2013) Obesity and colorectal cancer. Gut 62(6):933–947. https://doi.org/10.1136/gutjnl-2013-304701
Chen H, Zheng X, Zong X et al (2021) Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 70(6):1147–1154. https://doi.org/10.1136/gutjnl-2020-321661
Palmer TM, Lawlor DA, Harbord RM et al (2012) Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res 21(3):223–242. https://doi.org/10.1177/0962280210394459
Papadimitriou N, Dimou N, Tsilidis KK et al (2020) Physical activity and risks of breast and colorectal cancer: a Mendelian randomisation analysis. Nat Commun 11(1):597. https://doi.org/10.1038/s41467-020-14389-8
Burgess S, Thompson SG (2011) Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 40(3):755–764. https://doi.org/10.1093/ije/dyr036
Sanderson E, Spiller W, Bowden J (2021) Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat Med 40(25):5434–5452. https://doi.org/10.1002/sim.9133
Burgess S, Thompson SG (2017) Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol 32(5):377–389. https://doi.org/10.1007/s10654-017-0255-x
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44(2):512–525. https://doi.org/10.1093/ije/dyv080
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40(4):304–314. https://doi.org/10.1002/gepi.21965
Rees JMB, Wood AM, Burgess S (2017) Extending the MR-Egger method for multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat Med 36(29):4705–4718. https://doi.org/10.1002/sim.7492
Cohen JF, Chalumeau M, Cohen R, Korevaar DA, Khoshnood B, Bossuyt PMM (2015) Cochran’s Q test was useful to assess heterogeneity in likelihood ratios in studies of diagnostic accuracy. J Clin Epidemiol 68(3):299–306. https://doi.org/10.1016/j.jclinepi.2014.09.005
Hemani G, Bowden J, Davey SG (2018) Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet 27(R2):R195–R208. https://doi.org/10.1093/hmg/ddy163
Brion M-JA, Shakhbazov K, Visscher PM (2013) Calculating statistical power in Mendelian randomization studies. Int J Epidemiol 42(5):1497–1501. https://doi.org/10.1093/ije/dyt179
Hemani G, Zheng J, Elsworth B et al (2018) The MR-Base platform supports systematic causal inference across the human phenome. Elife. https://doi.org/10.7554/eLife.34408
Yavorska OO, Burgess S (2017) Mendelian randomization: an R package for performing Mendelian randomization analyses using summarized data. Int J Epidemiol 46(6):1734–1739. https://doi.org/10.1093/ije/dyx034
Hemani G, Tilling K, Davey SG (2017) Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet 13(11):e1007081. https://doi.org/10.1371/journal.pgen.1007081
Richmond RC, Davey SG (2019) Commentary: Orienting causal relationships between two phenotypes using bidirectional Mendelian randomization. Int J Epidemiol 48(3):907–911. https://doi.org/10.1093/ije/dyz149
Xiao G, He Q, Liu L et al (2022) Causality of genetically determined metabolites on anxiety disorders: a two-sample Mendelian randomization study. J Transl Med 20(1):475. https://doi.org/10.1186/s12967-022-03691-2
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K (2016) Body fatness and cancer-viewpoint of the IARC working group. N Engl J Med 375(8):794–798. https://doi.org/10.1056/NEJMsr1606602
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (London, England) 371(9612):569–578. https://doi.org/10.1016/S0140-6736(08)60269-X
Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M (2019) Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism 92:121–135. https://doi.org/10.1016/j.metabol.2018.11.001
Blücher C, Stadler SC (2017) Obesity and breast cancer: current insights on the role of fatty acids and lipid metabolism in promoting breast cancer growth and progression. Front Endocrinol 8:293. https://doi.org/10.3389/fendo.2017.00293
Sierra-Johnson J, Fisher RM, Romero-Corral A et al (2009) Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: findings from a multi-ethnic US population. Eur Heart J 30(6):710–717. https://doi.org/10.1093/eurheartj/ehn347
Fernández-Friera L, Fuster V, López-Melgar B et al (2017) Normal LDL-cholesterol levels are associated with subclinical atherosclerosis in the absence of risk factors. J Am Coll Cardiol 70(24):2979–2991. https://doi.org/10.1016/j.jacc.2017.10.024
Mach F, Baigent C, Catapano AL et al (2020) 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 41(1):111–188. https://doi.org/10.1093/eurheartj/ehz455
Kimak E, Nurczyk K, Skoczylas T, Duma D, Gieroba R, Solski J (2019) Fibroblast growth factor 21, epidermal growth factor receptor, interleukin 6, myeloperoxidase, lipid hydroperoxide, apolipoproteins A-I and B, as well as lipid and lipoprotein ratios as diagnostic serum biomarkers for gastric cancer. Pol Arch Intern Med 129(7–8):559–562. https://doi.org/10.20452/pamw.14836
Zhang X, Zhao X-W, Liu D-B et al (2014) Lipid levels in serum and cancerous tissues of colorectal cancer patients. World J Gastroenterol 20(26):8646–8652. https://doi.org/10.3748/wjg.v20.i26.8646
Ren L, Yi J, Li W et al (2019) Apolipoproteins and cancer. Cancer Med 8(16):7032–7043. https://doi.org/10.1002/cam4.2587
Yan X, Yao M, Wen X et al (2019) Elevated apolipoprotein B predicts poor postsurgery prognosis in patients with hepatocellular carcinoma. Onco Targets Ther 12:1957–1964. https://doi.org/10.2147/OTT.S192631
Nakajima K, Nagamine T, Fujita MQ, Ai M, Tanaka A, Schaefer E (2014) Apolipoprotein B-48: a unique marker of chylomicron metabolism. Adv Clin Chem 64:117–177
Yuasa-Kawase M, Masuda D, Kitazume-Taneike R et al (2012) Apolipoprotein B-48 to triglyceride ratio is a novel and useful marker for detection of type III hyperlipidemia after antihyperlipidemic intervention. J Atheroscler Thromb 19(9):862–871
Masuda D, Sakai N, Sugimoto T et al (2011) Fasting serum apolipoprotein B-48 can be a marker of postprandial hyperlipidemia. J Atheroscler Thromb 18(12):1062–1070
Pei W-d, Sun Y-h, Lu B et al (2007) Apolipoprotein B is associated with metabolic syndrome in Chinese families with familial combined hyperlipidemia, familial hypertriglyceridemia and familial hypercholesterolemia. Int J Cardiol 116(2):194–200
Ramasamy I (2014) Recent advances in physiological lipoprotein metabolism. Clin Chem Lab Med 52(12):1695–1727. https://doi.org/10.1515/cclm-2013-0358
Martin-Perez M, Urdiroz-Urricelqui U, Bigas C, Benitah SA (2022) The role of lipids in cancer progression and metastasis. Cell Metab 34(11):1675–1699. https://doi.org/10.1016/j.cmet.2022.09.023
Rozeveld CN, Johnson KM, Zhang L, Razidlo GL (2020) KRAS controls pancreatic cancer cell lipid metabolism and invasive potential through the lipase HSL. Cancer Res 80(22):4932–4945. https://doi.org/10.1158/0008-5472.CAN-20-1255
Li C, Wang Y, Liu D et al (2022) Squalene epoxidase drives cancer cell proliferation and promotes gut dysbiosis to accelerate colorectal carcinogenesis. Gut 71(11):2253–2265. https://doi.org/10.1136/gutjnl-2021-325851
Jun SY, Brown AJ, Chua NK et al (2021) Reduction of squalene epoxidase by cholesterol accumulation accelerates colorectal cancer progression and metastasis. Gastroenterology. https://doi.org/10.1053/j.gastro.2020.09.009
Alicandro G, Tavani A, La Vecchia C (2017) Coffee and cancer risk: a summary overview. Eur J Cancer Prev 26(5):424–432. https://doi.org/10.1097/CEJ.0000000000000341
Bradbury KE, Murphy N, Key TJ (2020) Diet and colorectal cancer in UK Biobank: a prospective study. Int J Epidemiol 49(1):246–258. https://doi.org/10.1093/ije/dyz064
Lee KJ, Choi JH, Jeong HG (2007) Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol 45(11):2118–2125
Hori A, Kasai H, Kawai K et al (2014) Coffee intake is associated with lower levels of oxidative DNA damage and decreasing body iron storage in healthy women. Nutr Cancer 66(6):964–969. https://doi.org/10.1080/01635581.2014.932398
(2002) Risk factors for atrophic chronic gastritis in a European population: results of the Eurohepygast study. Gut 50(6):779–785
Frondelius K, Borg M, Ericson U, Borné Y, Melander O, Sonestedt E (2017) Lifestyle and dietary determinants of serum apolipoprotein A1 and apolipoprotein B concentrations: cross-sectional analyses within a Swedish cohort of 24,984 individuals. Nutrients. https://doi.org/10.3390/nu9030211
Webb RJ, Mazidi M, Lip GYH, Kengne AP, Banach M, Davies IG (2022) The role of adiposity, diet and inflammation on the discordance between LDL-C and apolipoprotein B. Nutr Metab Cardiovasc Dis 32(3):605–615. https://doi.org/10.1016/j.numecd.2021.12.004
Davidson MH (2018) Triglyceride-rich lipoprotein cholesterol (TRL-C): the ugly stepsister of LDL-C. Eur Heart J 39(7):620–622. https://doi.org/10.1093/eurheartj/ehx741
Sun CJ, Brisson D, Gaudet D, Ooi TC (2020) Relative effect of hypertriglyceridemia on non-HDLC and apolipoprotein B as cardiovascular disease risk markers. J Clin Lipidol 14(6):825–836. https://doi.org/10.1016/j.jacl.2020.09.006
Chen Y, Liu L, Wang X et al (2013) Body mass index and risk of gastric cancer: a meta-analysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev 22(8):1395–1408. https://doi.org/10.1158/1055-9965.EPI-13-0042
Yang P, Zhou Y, Chen B et al (2009) Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer 45(16):2867–2873. https://doi.org/10.1016/j.ejca.2009.04.019
Chong P-K, Lee H, Zhou J et al (2010) Reduced plasma APOA1 level is associated with gastric tumor growth in MKN45 mouse xenograft model. J Proteomics 73(8):1632–1640. https://doi.org/10.1016/j.jprot.2010.04.005
Acknowledgements
All authors are grateful of investigators and participants of the UK Biobank and FinnGen biobank. We also sincerely thank the developers of Phenoscanner V2, R package “TwoSampleMR”, R package “MendelianRandomization”, and R package “MVMR”.
Funding
This research was funded by the Major Science and Technology Project of Liaoning Province, grant number 2022JH1/10400002.
Author information
Authors and Affiliations
Contributions
XL wrote the original draft; HY visualized the result; GY collected the data; BX edited the figures and tables; MS and MF revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics statement
We used publicly available summary data where no ethical approval is required.
Supplementary Information
Below is the link to the electronic supplementary material.
394_2023_3281_MOESM1_ESM.pdf
Supplementary file1 (PDF 21 KB) Supplementary Fig. 1 Forest plot of the casual effect of apolipoprotein B on gastric cancer
394_2023_3281_MOESM2_ESM.pdf
Supplementary file2 (PDF 20 KB) Supplementary Fig. 2 Forest plot of the casual effect of apolipoprotein B on colorectal cancer
394_2023_3281_MOESM3_ESM.pdf
Supplementary file3 (PDF 21 KB) Supplementary Fig. 3 Forest plot of the casual effect of apolipoprotein B on esophageal cancer
394_2023_3281_MOESM5_ESM.pdf
Supplementary file5 (PDF 8 KB) Supplementary Fig. 5 Forest plot of the casual effect of coffee intake on colorectal cancer
394_2023_3281_MOESM6_ESM.pdf
Supplementary file6 (PDF 8 KB) Supplementary Fig. 6 Forest plot of the casual effect of coffee intake on esophageal cancer
394_2023_3281_MOESM7_ESM.pdf
Supplementary file7 (PDF 5 KB) Supplementary Fig. 7 Scatter plot of the causal effect of gastric cancer on apolipoprotein B
394_2023_3281_MOESM8_ESM.pdf
Supplementary file8 (PDF 5 KB) Supplementary Fig. 8 Scatter plot of the causal effect of gastric cancer on coffee intake
394_2023_3281_MOESM9_ESM.pdf
Supplementary file9 (PDF 5 KB) Supplementary Fig. 9 Scatter plot of the causal effect of colorectal cancer on apolipoprotein B
394_2023_3281_MOESM10_ESM.pdf
Supplementary file10 (PDF 5 KB) Supplementary Fig. 10 Scatter plot of the causal effect of colorectal cancer on coffee intake
394_2023_3281_MOESM11_ESM.pdf
Supplementary file11 (PDF 5 KB) Supplementary Fig. 11 Scatter plot of the causal effect of esophageal cancer on apolipoprotein B
394_2023_3281_MOESM12_ESM.pdf
Supplementary file12 (PDF 5 KB) Supplementary Fig. 12 Scatter plot of the causal effect of esophageal cancer on coffee intake
394_2023_3281_MOESM13_ESM.pdf
Supplementary file13 (PDF 5 KB) Supplementary Fig. 13 Funnel plot of the casual effect of gastric cancer on apolipoprotein B
394_2023_3281_MOESM14_ESM.pdf
Supplementary file14 (PDF 5 KB) Supplementary Fig. 14 Funnel plot of the casual effect of gastric cancer on coffee intake
394_2023_3281_MOESM15_ESM.pdf
Supplementary file15 (PDF 5 KB) Supplementary Fig. 15 Funnel plot of the casual effect of colorectal cancer on apolipoprotein B
394_2023_3281_MOESM16_ESM.pdf
Supplementary file16 (PDF 5 KB) Supplementary Fig. 16 Funnel plot of the casual effect of colorectal cancer on coffee intake
394_2023_3281_MOESM17_ESM.pdf
Supplementary file17 (PDF 5 KB) Supplementary Fig. 17 Funnel plot of the casual effect of esophageal cancer on apolipoprotein B
394_2023_3281_MOESM18_ESM.pdf
Supplementary file18 (PDF 5 KB) Supplementary Fig. 18 Funnel plot of the casual effect of esophageal cancer on coffee intake
394_2023_3281_MOESM19_ESM.pdf
Supplementary file19 (PDF 5 KB) Supplementary Fig. 19 Forest plot of the casual effect of gastric cancer on apolipoprotein B
394_2023_3281_MOESM20_ESM.pdf
Supplementary file20 (PDF 5 KB) Supplementary Fig. 20 Forest plot of the casual effect of gastric cancer on coffee intake
394_2023_3281_MOESM21_ESM.pdf
Supplementary file21 (PDF 5 KB) Supplementary Fig. 21 Forest plot of the casual effect of colorectal cancer on apolipoprotein B
394_2023_3281_MOESM22_ESM.pdf
Supplementary file22 (PDF 5 KB) Supplementary Fig. 22 Forest plot of the casual effect of colorectal cancer on coffee intake
394_2023_3281_MOESM23_ESM.pdf
Supplementary file23 (PDF 5 KB) Supplementary Fig. 23 Forest plot of the casual effect of esophageal cancer on apolipoprotein B
394_2023_3281_MOESM24_ESM.pdf
Supplementary file24 (PDF 5 KB) Supplementary Fig. 24 Forest plot of the casual effect of esophageal cancer on coffee intake
394_2023_3281_MOESM25_ESM.pdf
Supplementary file25 (PDF 20 KB) Supplementary Fig. 25 Leave-one-out inverse-variance weighted mendelian randomization analysis of apolipoprotein B on gastric cancer
394_2023_3281_MOESM26_ESM.pdf
Supplementary file26 (PDF 20 KB) Supplementary Fig. 26 Leave-one-out inverse-variance weighted mendelian randomization analysis of apolipoprotein B on colorectal cancer
394_2023_3281_MOESM27_ESM.pdf
Supplementary file27 (PDF 20 KB) Supplementary Fig. 27 Leave-one-out inverse-variance weighted mendelian randomization analysis of apolipoprotein B on esophageal cancer
394_2023_3281_MOESM28_ESM.pdf
Supplementary file28 (PDF 8 KB) Supplementary Fig. 28 Leave-one-out inverse-variance weighted mendelian randomization analysis of coffee intake on gastric cancer
394_2023_3281_MOESM29_ESM.pdf
Supplementary file29 (PDF 8 KB) Supplementary Fig. 29 Leave-one-out inverse-variance weighted mendelian randomization analysis of coffee intake on colorectal cancer
394_2023_3281_MOESM30_ESM.pdf
Supplementary file30 (PDF 8 KB) Supplementary Fig. 30 Leave-one-out inverse-variance weighted mendelian randomization analysis of coffee intake on esophageal cancer
394_2023_3281_MOESM31_ESM.pdf
Supplementary file31 (PDF 5 KB) Supplementary Fig. 31 Leave-one-out inverse-variance weighted mendelian randomization analysis of gastric cancer on apolipoprotein B
394_2023_3281_MOESM32_ESM.pdf
Supplementary file32 (PDF 5 KB) Supplementary Fig. 32 Leave-one-out inverse-variance weighted mendelian randomization analysis of gastric cancer on coffee intake
394_2023_3281_MOESM33_ESM.pdf
Supplementary file33 (PDF 5 KB) Supplementary Fig. 33 Leave-one-out inverse-variance weighted mendelian randomization analysis of colorectal cancer on apolipoprotein B
394_2023_3281_MOESM34_ESM.pdf
Supplementary file34 (PDF 5 KB) Supplementary Fig. 34 Leave-one-out inverse-variance weighted mendelian randomization analysis of colorectal cancer on coffee intake
394_2023_3281_MOESM35_ESM.pdf
Supplementary file35 (PDF 5 KB) Supplementary Fig. 35 Leave-one-out inverse-variance weighted mendelian randomization analysis of esophageal cancer on apolipoprotein B
394_2023_3281_MOESM36_ESM.pdf
Supplementary file36 (PDF 5 KB) Supplementary Fig. 36 Leave-one-out inverse-variance weighted mendelian randomization analysis of esophageal cancer on coffee intake
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Yu, H., Yan, G. et al. Causal relationships between coffee intake, apolipoprotein B and gastric, colorectal, and esophageal cancers: univariable and multivariable Mendelian randomization. Eur J Nutr 63, 469–483 (2024). https://doi.org/10.1007/s00394-023-03281-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03281-y