Abstract
Purpose
Bacillus coagulans GBI-30, 6086 (BC30) was previously shown to improve nutrient digestibility and amino acid absorption from milk protein in vitro. However, the effect of supplementation with this probiotic on lactose digestibility has not yet been evaluated in vivo.
Methods
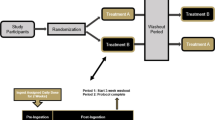
Wistar female rats were exposed to an acute high-lactose diet (LD; 35% lactose) meal challenge after 7 days of administration of BC30 (LD-BC; n = 10) or vehicle (LD-C; n = 10). Rats treated with vehicle and exposed to control diet (CD; 35% corn starch) meal were used as controls (CD-C; n = 10). Carbohydrate oxidation (CH_OX) and lipid oxidation (L_OX) were monitored by indirect calorimetry before and after lactose challenge. After the challenge, rats were treated daily with vehicle or probiotic for an additional week and were fed with CD or LD ad libitum to determine the effects of BC30 administration in a lactose-induced diarrhoea and malnutrition model.
Results
LD-C rats showed lower CH_OX levels than CD rats, while LD-BC rats showed similar CH_OX levels compared to CD rats during the lactose challenge, suggesting a better digestion of lactose in the rats supplemented with BC30. BC30 completely reversed the increase in the small intestine length of LD-C animals. LD-BC rats displayed increased intestinal mRNA Muc2 expression. No significant changes were observed due to BC30 administration in other parameters, such as serum calprotectin, intestinal MPO activity, intestinal A1AT and SGLT1 levels or intestinal mRNA levels of Claudin2 and Occludin.
Conclusion
Treatment with BC30 improved the digestibility of lactose in an acute lactose challenge and ameliorated some of the parameters associated with lactose-induced malnutrition.




Similar content being viewed by others
Data availability
The datasets used and analysed in this current study are available from the corresponding author (JT) upon reasonable request.
References
Ugidos-Rodríguez S, Matallana-González MC, Sánchez-Mata MC (2018) Lactose malabsorption and intolerance: a review. Food Funct 9:4056–4068. https://doi.org/10.1039/C8FO00555A
Silanikove N, Leitner G, Merin U (2015) The interrelationships between lactose intolerance and the modern dairy industry: global perspectives in evolutional and historical backgrounds. Nutrients 7:7312. https://doi.org/10.3390/NU7095340
Chen L, Tuo B, Dong H (2016) Regulation of intestinal glucose absorption by ion channels and transporters. Nutrients. https://doi.org/10.3390/NU8010043
Misselwitz B, Butter M, Verbeke K, Fox MR (2019) Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut 68:2080–2091. https://doi.org/10.1136/GUTJNL-2019-318404
Yang J, Deng Y, Chu H et al (2013) Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. https://doi.org/10.1016/J.CGH.2012.11.034
Ibba I, Gilli A, Boi MF, Usai P (2014) Effects of exogenous lactase administration on hydrogen breath excretion and intestinal symptoms in patients presenting lactose malabsorption and intolerance. Biomed Res Int. https://doi.org/10.1155/2014/680196
Oak SJ, Jha R (2019) The effects of probiotics in lactose intolerance: a systematic review. Crit Rev Food Sci Nutr 59:1675–1683. https://doi.org/10.1080/10408398.2018.1425977
Maathuis AJH, Keller D, Farmer S (2010) Survival and metabolic activity of the GanedenBC30 strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Benef Microbes 1:31–36. https://doi.org/10.3920/BM2009.0009
Tripathi MK, Giri SK (2014) Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods 9:225–241. https://doi.org/10.1016/J.JFF.2014.04.030
Dolin BJ (2009) Effects of a proprietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods Find Exp Clin Pharmacol 31:655–659. https://doi.org/10.1358/MF.2009.31.10.1441078
Kalman DS, Schwartz HI, Alvarez P et al (2009) A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol 9:85. https://doi.org/10.1186/1471-230X-9-85
Jensen GS, Benson KF, Carter SG, Endres JR (2010) GanedenBC30™ cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC Immunol 11:15. https://doi.org/10.1186/1471-2172-11-15
Kimmel M, Keller D, Farmer S, Warrino DE (2010) A controlled clinical trial to evaluate the effect of GanedenBC(30) on immunological markers. Methods Find Exp Clin Pharmacol 32:129–132. https://doi.org/10.1358/MF.2010.32.2.1423881
Fitzpatrick LR, Small JS, Greene WH et al (2012) Bacillus coagulans GBI-30, 6086 limits the recurrence of Clostridium difficile-induced colitis following vancomycin withdrawal in mice. Gut Pathogens. https://doi.org/10.1186/1757-4749-4-13
Stecker RA, Moon JM, Russo TJ et al (2020) Bacillus coagulans GBI-30, 6086 improves amino acid absorption from milk protein. Nutr Metab (Lond). https://doi.org/10.1186/S12986-020-00515-2
Courtois P, Sener A, Scott FW, Malaisse WJ (2004) Disaccharidase activity in the intestinal tract of Wistar-Furth, diabetes-resistant and diabetes-prone biobreeding rats. Br J Nutr 91:201–209. https://doi.org/10.1079/BJN20041026
Norton R, Leite J, Vieira E et al (2001) Use of nucleotides in weanling rats with diarrhea induced by a lactose overload: effect on the evolution of diarrhea and weight and on the histopathology of intestine, liver and spleen. Braz J Med Biol Res 34:195–202
Boakye PA, Brierley SM, Pasilis SP, Balemba OB (2012) Garcinia buchananii bark extract is an effective anti-diarrheal remedy for lactose-induced diarrhea. J Ethnopharmacol 142:539–547. https://doi.org/10.1016/j.jep.2012.05.034
Arciniegas EL, Cioccia AMHP (2000) Effect of the lactose induced diarrhea on macronutrients availability and immune function in well-nourished and undernourished rats. Arch Latinoam Nutr 50:48–54
Bueno J, Torres M, Almendros A et al (1994) Effect of dietary nucleotides on small intestinal repair after diarrhoea. Histological and ultrastructural changes. Gut 35:926–933. https://doi.org/10.1136/gut.35.7.926
Alexandre V, Even PC, Larue-Achagiotis C et al (2013) Lactose malabsorption and colonic fermentations alter host metabolism in rats. Br J Nutr 110:625–631. https://doi.org/10.1017/S0007114512005557
Alexandre V, Davila AM, Bouchoucha M et al (2013) Agreement between indirect calorimetry and traditional tests of lactose malabsorption. Dig Liver Dis 45:727–732. https://doi.org/10.1016/J.DLD.2013.03.015
Weir JBV (1949) New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 109:1–9. https://doi.org/10.1113/JPHYSIOL.1949.SP004363
Frayn KN (1983) Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55:628–634
Carraro F, Stuart CA, Hartl WH et al (1990) Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol 259:E470–E476
Bircher S, Knechtle B (2004) Relationship between fat oxidation and lactate threshold in athletes and obese women and men. J Sport Sci Med 3:174–181
Sung J, Morales W, Kim G et al (2013) Effect of repeated Campylobacter jejuni infection on gut flora and mucosal defense in a rat model of post infectious functional and microbial bowel changes. Neurogastroenterol Motil 25:529–537. https://doi.org/10.1111/nmo.12118
Abdin AA (2013) Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol 718:145–153. https://doi.org/10.1016/J.EJPHAR.2013.08.040
Bradley PP, Priebat DA, Christensen RD, Rothstein G (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 78:206–209. https://doi.org/10.1111/1523-1747.ep12506462
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Nieto N, Lopez-Pedrosa JM, Mesa MD et al (2000) Chronic diarrhea impairs intestinal antioxidant defense system in rats at weaning. Dig Dis Sci 45:2044–2050. https://doi.org/10.1023/A:1005603019800
van de Heijning BJM, Kegler D, Schipper L et al (2015) Acute and chronic effects of dietary lactose in adult rats are not explained by residual intestinal lactase activity. Nutrients 7:5542–5555. https://doi.org/10.3390/NU7075237
Collares EF, Rossi MA, Macedo AS (1985) Experimental dilatation of the cecum and colon in rats. II. Reversion after induction by the continuous administration of lactose. Arq Gastroenterol 22:192–195
Kim KI, Benevenga NJ, Grummer RH (1979) In vitro measurement of the lactase activity and the fermentation products of lactose in the cecal and colonic contents of rats fed a control or 30% lactose diet. J Nutr 109:856–863. https://doi.org/10.1093/JN/109.5.856
Poulsen SB, Fenton RA, Rieg T (2015) Sodium-glucose cotransport. Curr Opin Nephrol Hypertens 24:463–469
Gisbert JP, McNicholl AG (2009) Questions and answers on the role of faecal calprotectin as a biological marker in inflammatory bowel disease. Dig Liver Dis 41:56–66. https://doi.org/10.1016/J.DLD.2008.05.008
Nanini HF, Bernardazzi C, Castro F, De Souza HSP (2018) Damage-associated molecular patterns in inflammatory bowel disease: from biomarkers to therapeutic targets. World J Gastroenterol 24:4622–4634. https://doi.org/10.3748/WJG.V24.I41.4622
Lehmann FS, Burri E, Beglinger C (2015) The role and utility of faecal markers in inflammatory bowel disease. Ther Adv Gastroenterol 8:23–36. https://doi.org/10.1177/1756283X14553384
Kalla R, Kennedy NA, Ventham NT et al (2016) Serum calprotectin: a novel diagnostic and prognostic marker in inflammatory bowel diseases. Am J Gastroenterol 111:1796–1805. https://doi.org/10.1038/AJG.2016.342
Khan AA, Alsahli MA, Rahmani AH (2018) Myeloperoxidase as an active disease biomarker: recent biochemical and pathological perspectives. Med Sci (Basel, Switzerland). https://doi.org/10.3390/MEDSCI6020033
Celi P, Verlhac V, Pérez Calvo E et al (2019) Biomarkers of gastrointestinal functionality in animal nutrition and health. Anim Feed Sci Technol 250:9–31. https://doi.org/10.1016/J.ANIFEEDSCI.2018.07.012
Günzel D, Yu ASL (2013) Claudins and the modulation of tight junction permeability. Physiol Rev 93:525–569. https://doi.org/10.1152/physrev.00019.2012
Lee SH (2015) Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res 13:11. https://doi.org/10.5217/ir.2015.13.1.11
Venugopal S, Anwer S, Szászi K (2019) Claudin-2: roles beyond permeability functions. Int J Mol Sci 20:5655
Corfield AP (2015) Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim Biophys Acta 1850:236–252. https://doi.org/10.1016/J.BBAGEN.2014.05.003
Ma S, Yeom J, Lim YH (2020) Dairy Propionibacterium freudenreichii ameliorates acute colitis by stimulating MUC2 expression in intestinal goblet cell in a DSS-induced colitis rat model. Sci Rep. https://doi.org/10.1038/S41598-020-62497-8
Gao J, Li Y, Wan Y et al (2019) A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front Microbiol. https://doi.org/10.3389/FMICB.2019.00477
Yu JY, He XL, Puthiyakunnon S et al (2015) Mucin2 is required for probiotic agents-mediated blocking effects on meningitic E. coli-induced pathogenicities. J Microbiol Biotechnol 25:1751–1760. https://doi.org/10.4014/JMB.1502.02010
Kuugbee ED, Shang X, Gamallat Y et al (2016) Structural change in microbiota by a probiotic cocktail enhances the gut barrier and reduces cancer via TLR2 signaling in a rat model of colon cancer. Dig Dis Sci 61:2908–2920. https://doi.org/10.1007/S10620-016-4238-7
Cervantes-Garciá D, Jiménez M, Rivas-Santiago CE et al (2021) Lactococcus lactis NZ9000 prevents asthmatic airway inflammation and remodelling in rats through the improvement of intestinal barrier function and systemic TGF-β production. Int Arch Allergy Immunol 182:277–291. https://doi.org/10.1159/000511146
Zhuge A, Li B, Yuan Y et al (2020) Lactobacillus salivarius LI01 encapsulated in alginate-pectin microgels ameliorates d-galactosamine-induced acute liver injury in rats. Appl Microbiol Biotechnol 104:7437–7455. https://doi.org/10.1007/S00253-020-10749-Y
Acknowledgements
We would like to express our thanks for their technical support to Yaiza Tobajas, Anna Antolín, Iris Triguero and Cristina Egea, who are laboratory technicians at the Technological Unit of Nutrition and Health.
Funding
This work was financially supported by the Centre for the Development of Industrial Technology (CDTI) of the Spanish Ministry of Science and Innovation under grant agreement of a project called TOLERA EXP 00101692.
Author information
Authors and Affiliations
Contributions
Conceptualization, JT, JMDB, AC, FP, AR-G, AH and VG; funding acquisition, JMDB, AC and FP; investigation, JT, UC, RM-C, CD-C, AM-C, JMA-H and GC; formal analysis, JT and UC; methodology, JT and RM-C; writing—original draft, JT; writing—review and editing, JT, UC, RM-C, CD-C, JMA-H, JMDB and AC; supervision, JMDB and AC.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teichenné, J., Catalán, Ú., Mariné-Casadó, R. et al. Bacillus coagulans GBI-30, 6086 (BC30) improves lactose digestion in rats exposed to a high-lactose meal. Eur J Nutr 62, 2649–2659 (2023). https://doi.org/10.1007/s00394-023-03183-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03183-z