Abstract
Purpose
Bile acid (BA) metabolism by intestinal bacteria is associated with the risk of gastrointestinal diseases; additionally, its control has become a modern strategy for treating metabolic diseases. This cross-sectional study investigated the influence of defecation status, intestinal microbiota, and habitual diet on fecal BA composition in 67 community-dwelling young participants.
Methods
Feces were collected for intestinal microbiota and BA analyses; data about defecation status and dietary habits were collected using the Bristol stool form scales and a brief-type self-administered diet history questionnaire, respectively. The participants were categorized into four clusters based on their fecal BA composition, according to cluster analysis, and tertiles based on deoxycholic acid (DCA) and lithocholic acid (LCA) levels.
Results
The high primary BA (priBA) cluster with high fecal cholic acid (CA) and chenodeoxycholic acid (CDCA) levels had the highest frequency of normal feces, whereas the second BA (secBA) cluster with high levels of fecal DCA and LCA had the lowest. Alternately, the high-priBA cluster had a distinct intestinal microbiota, with higher Clostridium subcluster XIVa and lower Clostridium cluster IV and Bacteroides. The low-secBA cluster with low fecal DCA and LCA levels had the lowest animal fat intake. Nevertheless, the insoluble fiber intake of the high-priBA cluster was significantly higher than that of the high-secBA cluster.
Conclusion
High fecal CA and CDCA levels were associated with distinct intestinal microbiota. Conversely, high levels of cytotoxic DCA and LCA were associated with increased animal fat intake and decreased frequency of normal feces and insoluble fiber intake.
Clinical trial registry
University Hospital Medical Information Network (UMIN) Center system (UMIN000045639); date of registration: 15/11/2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary bile acids (priBAs)—including cholic (CA) and chenodeoxycholic acids (CDCA)—are synthesized from cholesterol in the human liver, conjugated with either taurine or glycine, and secreted into the intestinal tract, where they dissolve dietary lipids [1]. Conjugated priBAs are then reabsorbed in the terminal ileum and returned to the liver [2]. Some conjugated priBAs are modified by intestinal bacteria in a stepwise manner [3]. Starting with a deconjugation of priBAs to free priBAs by intestinal bacteria with the aid of bile salt hydrolase activity [4], the free priBAs are then converted to various secondary BAs (secBAs) [2]. Intestinal BA metabolism is associated with the incidence and progression of various diseases [5, 6]. Thus, modifying the intestinal BA metabolism could emerge as a new strategy for preventing or treating several diseases [7].
Intestinal bacteria converted CA and CDCA via 7α-dehydroxylation into deoxycholic acid (DCA) and lithocholic acid (LCA), respectively, which are the predominant BAs in human large bowel [2]. DCA and LCA are potentially genotoxic and tumor-promoting [8]. Their increased production has been associated with a similarly increased risk of colon cancer [9], cholelithiasis [10], and liver cancer [11]. Alternatively, as BAs help regulate lipid and glucose metabolism via the farnesoid X receptor, use of antibiotics to inhibit secBA production reduced serum triglyceride and glucose levels [12]. Probiotics may also help lower serum cholesterol levels [13, 14] due to increased fecal excretion. This is accomplished through increased free priBA production and low reabsorption in the terminal ileum [15]. However, this theory is controversial because free priBAs might be further converted to toxic secBAs within the colon [16]. Additionally, increased exposure to BAs in the colon might be associated with diarrhea by enhancing the secretion of fluids and electrolytes [17]. On the other hand, low levels of Bacteroidetes, bacteria that predominantly produce bile salt hydrolase [18], have been found in patients with obesity [19]. Additionally, low levels of Clostridium subcluster XIVa, to which bacteria with 7α-dehydroxylating activity belong, are associated with intestinal dysbiosis [20]. Although BAs are modified by intestinal bacteria, it remains unclear how the relative abundance of various strains of bacteria affect human health [21].
Diet is a modifiable factor that can influence defecation status [22], BAs [23], and intestinal microbiota [24, 25]. Defecation status, BAs, and intestinal microbiota may interact [17, 20, 26, 27]. Given known associations between various BAs and disease prevention or treatment, we sought to clarify the relationships that exist among diet, defecation status, BAs, and intestinal microbiota. Unfortunately, few studies have examined all these factors. Additionally, BA studies conducted with animal models are limited by known differences between humans and non-human animals [28]. Specifically, the main priBAs in rats are CA and β-muricholic acid; whereas, in humans the main priBAs are CA and CDCA. Thus, human studies may be more relevant. This cross-sectional study identified associations among defecation status, intestinal microbiota, and diet by examining fecal BA composition in community-dwelling young participants.
Methods
Study participants
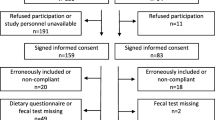
The study participants were 70 students enrolled at the Yamagata Prefectural Yonezawa University of Nutrition Sciences or Sakura no Seibo Junior College. Two participants who had taken medications for diarrhea or constipation during the week before fecal sampling were excluded from the analysis. Additionally, during cluster analysis, we excluded a third participant who did not fit any cluster. Therefore, 67 participants were included in the analysis (5 males, 62 females; age: 18–22). All participants provided written informed consent; the study protocol was approved by the Ethics Committee of the Yamagata Prefectural Yonezawa University of Nutrition Sciences (Approval No. 2019–9), and the study protocol was according to the Declaration of Helsinki.
Protocol
The participants underwent the following examinations one time each: (A) recording of defecation status and probiotic foods consumed the week before the fecal collection, (B) fecal collection, (C) dietary assessment using a brief, self-administered diet history questionnaire (BDHQ) [29, 30]. The participants were instructed to carry on with their typical routines while in the study and not make any major changes to their general diet or physical activity.
Records of defecation status and probiotic foods
The participants recorded their defecation status using the Bristol stool form scale (BSFS) for 1 week before fecal collection. BSFS is a tool designed to classify fecal form into seven categories (types 1–2 for hard feces, types 3–5 for normal feces, and types 6–7 for watery feces). This tool is widely used in clinical and research fields [31, 32]. The participants were instructed about the BSFS and asked to self-assess and record the form of their feces immediately after defecation. They were asked to also record the probiotic foods (defined as foods containing lactic acid bacteria and bifidobacteria) taken for this period. These records were kept until the feces were collected.
Collection and analysis of feces
We asked the participants to collect their feces at a convenient time after a week of recording their bowel movements and intake of probiotic foods. Following the instructions, the participants collected all defecated feces immediately in a special fecal collection cup, stirred them several times with a spoon, and then placed the collection cup in a container, which in turn, was set in a cool bag with the coolant. The participant also evaluated their collected feces using the BSFS. The participants submitted the bag to the researcher as soon as possible (i.e., samples collected in the early morning on weekdays were submitted in the morning; samples collected on campus were submitted promptly; if the samples were collected on a holiday or at night, the participant contacted the researcher, who then picked up the sample at the participant’s home). The submitted fecal samples were stored at − 80 °C until use.
We analyzed fecal microbiota by Techno Suruga Laboratory Co., Ltd. (Shizuoka, Japan), using terminal restriction fragment length polymorphism (T-RFLP) targeting the bacterial 16S rDNA. DNA were extracted from the fecal samples according to a previously published protocol [33]. Briefly, 100 mg of each fecal sample was suspended in 4 M guanidine thiocyanate, 100 mM Tris–HCl (pH 9.0), and 40 mM EDTA and then beaten with zirconia beads using a FastPrep-24 5G instrument (MP Biomedicals, USA) to obtain crude extracted DNA. The DNA was purified using an automated DNA isolation system (GENE PREP STAR PI-480, Kurabo Industries, Japan), and a DNA isolation reagent kit (NR-201, Kurabo Industries, Japan). We estimated DNA concentrations using the NanoDrop ND8000 (Thermo Ficher Scientific, USA) and adjusted the final DNA sample concentration to 10 ng/μL. We performed amplification of 16S rDNA, restriction enzyme digestion, and fragment analysis according to a previously published protocol [34, 35]. Here 16S rDNA was amplified using a fluorescent-labeled 516f primer (5′-TGCCAGCAGCCGCGGTA-3′) and 1510r primer (5′-GGTTACCTTGTTACGACTT-3′) The resulting 16S rDNA amplicons were digested with FastDigest BseLI (BslI, Thermo Fisher Scientific, USA) for 10 min. The digested products were subjected to fragment analysis via the ABI PRISM 3130xl Genetic Analyzer System (Applied Biosystems, USA). The taxonomy of the clostridial species was classified into clusters, as proposed by Collins et al. [36, 37].
We analyzed fecal BA concentrations by Techno Suruga Laboratory Co., Ltd. (Shizuoka, Japan) using liquid chromatography in combination with hybrid quadrupole time-of-flight mass spectrometry (LC-QTOF-MS). BAs were extracted from fecal samples using a method previously described [38] with minor modifications; 100 mg of each fecal sample were suspended in 0.9 mL of sodium acetate buffer (100 mM, pH 5.6) mixed with ethanol using a 2 mL tube with zirconia beads and then heat-treated at 85℃ for 30 min. After centrifugation at 18,400×g for 10 min, the supernatant was diluted fourfold with water and applied to the solid-phase extraction using a Bond Elut C18 cartridge (Agilent Technologies, USA). The solvent of the obtained extract was evaporated, and the residue was dissolved in 50% ethanol with internal standard. This solution was filtered through a hydrophilic polytetrafluoroethylene filter and used as a sample for LC-QTOF-MS analysis. The LC-QTOF-MS instrument comprises Waters ACQUITY UPLC, Xevo G2-S QTOF, and an electrospray ionization probe (waters, USA). An Acquity UPLC BEH C18 column (1.7 µm, 2.1 × 150 mm, Waters, USA) was used at 65℃. Gradient elution performed the separation using 0.1% formic acid aqueous solution (solvent A) and acetonitrile containing 0.1% formic acid (solvent B) at a flow rate of 0.5 mL/min. The gradient elution program for solvent B is as follows: 0–0.5 min, 30%; 0.5–1.0 min, 30–35%; 1.0–7.0 min, 35–40%; 7.0–10.0 min, 40–50%; 10.0–11.5 min, 50–95%; and 11.5–13.0 min, 95%. The QTOF mass spectrometer operated in negative ion mode. The desolvation gas was nitrogen, the collision gas was argon, and the following parameters were used: capillary voltage, 0.5 kV; sampling cone voltage, 20 V; source temperature, 150 ℃; desolvation temperature, 450 ℃; cone gas flow, 100 L/h; desolvation gas flow, 1000 L/h; scan time, 0.3 s; and data acquisition region, 50–850 m/z. Leucine enkephalin was used as lock mass, which generated a 554.2615 Da [M-H]- ion.
Assessment of habitual diet
We assessed participants’ diets during the preceding month using the BDHQ [29, 30]. This questionnaire, based primarily on the Standard Table of Food Composition in Japan and formulated by Japan MEXT [39], asks how frequently the respondent consumes 58 different foods and beverages. A commercial computer algorithm was used to calculate nutritional intake. Because the BDHQ is able to rank the energy-adjusted intake of many nutrients [29, 30], each participant’s consumption of various food items was expressed as density per 1000 kcal.
Categorization based on the fecal BAs composition
We categorized the participants of the study based on their fecal BAs composition by combining cluster analyses and tertiles. The 17 types of BA concentrations we analyzed were subjected to subsequent cluster analysis [including five free BAs (CA, CDCA, DCA, LCA, and ursodeoxycholic acid (UDCA)), five glycine conjugated (G-) BAs (G-CA, G-CDCA, G-DCA, G-LCA, and G-UDCA), five taurine conjugated (T-) BAs (T-CA, T-CDCA, T-DCA, T-LCA, and T-UDCA), 7-oxo-DCA, and 7-oxo-LCA]. The data matrix is presented as Online Resource 1. We used non-standardized variables because they were all on the same scale (µmol/g), and the data were comparable. First, we determined the number of clusters through the tree diagram, which is generated using the squared Euclidean distance via the ward’s method [40]. Thereafter, participants were categorized using K-means cluster analysis based on squared Euclidean distances. The K-means method is one of the most widely used clustering methods and requires the number of clusters to be set in advance [41]. Subsequently, one obtained cluster was further divided into tertiles based on the total concentrations of DCA and LCA.
The fecal bile acid levels were measured per fresh fecal mass and were therefore affected by the fecal water content. Thus, we analyzed the categorization scheme generated by the cluster analysis, which also included the BSFS type of feces used in the analysis as a variable. Two-step cluster analysis, which can also handle categorical variables, was used. All categories obtained by cluster analysis including the BSFS type were identical, with the exception of one participant’s classification (see Online Resource 2), and the results of subsequent analyses were similar. All cluster analyses were performed using the Statistical Package for the Social Sciences Software Ver. 28.0 for Windows (IBM SPSS 28 Statistics Base, Inc., Chicago, IL, USA).
Statistical analysis
Characteristics of the study participants were presented as mean ± standard deviation (SD) or percentage. Between-cluster differences were assessed using the one-way analysis of variance (ANOVA). We used Tukey’s test for post hoc pairwise multiple comparisons if Levene's test showed homogeneity of variance; the Games–Howell’s test was used for samples with non-homogeneous variances. The data are presented as mean ± SD. To better interpret the association of defecation status, intestinal microbiota, and habitual diet with fecal BA composition, we performed principal component analysis (PCA) on some variables that showed significant differences among clusters based on the fecal BAs composition. The variables used in the PCA were shown in Online Resources 3. All statistical analyses were performed using the Statistical Package for the Social Sciences Software Ver. 28.0 for Windows (IBM SPSS, Inc., Chicago, IL, USA); p values of < 0.05 are considered indicative of statistical significance.
Results
Characteristics of the participants
The characteristics of the participants are shown in Online Resource 4. The mean age was 19.9 years, with a disproportionate number of females (92.5%). Their mean BMI was 21.2 kg/m2, and 77.6% were within normal limits for body weight (18.5 ≤ BMI < 25). Participants’ defecation status is presented as Fig. 1; 80.6% of the participants had a mean BSFS score of 3 ~ 5 (normal feces) for one week (Fig. 1a). The mean ± SD of the frequency of normal feces was 6.7 ± 3.9 time/week (Fig. 1b); 50.7% and 73.1% of the participants had no hard and watery stools during the week, respectively (Fig. 1c, d).
Defecation status for a week in study young participants living in the community. The participants assessed and recorded all feces excreted in the week using the BSFS. a Distribution of weekly mean score of BSFS for each participant. b Distribution of frequency of excretion of normal feces (BSFS type 3–5). c Distribution of frequency of excretion of hard feces (BSFS type 1–2). d Distribution of frequency of excretion of watery feces (BSFS type 6–7). BSFS Bristol stool form scale
The distribution of total BA levels, according to fecal form, is shown in Fig. 2. The feces of 80.6% (n = 54) were considered normal, 16.4% (n = 11) hard, and 3.0% (n = 2) watery. There were no significant differences in fecal total bile acid levels relative to BSFS type (ANOVA, p = 0.499, data not shown).
Distribution of total bile acid levels according to the form of the analyzed feces. The form of the feces used in the analysis was assessed and recorded by the participant using the BSFS at the time of fecal collection. BSFS types 1 and 2 indicate hard stools, 3–5 indicate normal stools, and 6 and 7 indicate watery stools. BSFS Bristol stool form scale. 1Bile acid levels were measured per fresh fecal mass
Table 1 shows the participants’ fecal characteristics. Out of 17 BAs measured, DCA and LCA were predominant, with mean values of 1.57 and 0.89 µmol/g, respectively; 7-oxo-LCA, five G-BAs, and five T-BAs were detected only in the feces of two, four, and six participants, respectively. The Bacteroides, Clostridium cluster XIVa, and Bifidobacterium were predominant, with mean relative abundance values of 29.9%, 22.8%, and 19.4%, respectively.
Categorization of the participants
We used cluster analysis to categorize the participants based on the composition of their fecal BAs. Consequently, two clusters were generated, one with a high level of CA and CDCA (Fig. 3, cluster 4) labeled as high-priBA and the other (Fig. 3, clusters 1–3) predominated by DCA and LCA, and further divided into tertiles based on the total concentration of DCA and LCA, labeled as low-secBA, medium-secBA, and high-secBA, respectively. Consequently, the participants were divided into four clusters (Fig. 3).
Profiling of fecal bile acids of the participants living in the community. DCA deoxycholic acid, LCA lithocholic acid, CA cholic acid, CDCA chenodeoxycholic acid, UDCA ursodeoxycholic acid, G-BA glycine conjugated bile acid, T-BA taurine conjugated bile acid, priBA primary bile acid, secBA secondary bile acid. G-BA and T-BA each contained five bile acids (CA, CDCA, DCA, LCA, and UDCA). 1Bile acid levels were measured per fresh fecal mass
Differences in defecation status among the various BA clusters
Table 2 showed the differences in fecal BA levels and defecation status among BA clusters, where the levels of UDCA differed significantly (ANOVA, p = 0.034). The high-priBA cluster had the highest levels (Games–Howell’s test; low-secBA vs. high-priBA, p = 0.029; medium-secBA vs. high-priBA, p = 0.043; high-secBA vs. high-priBA, p = 0.054). On the other hand, the frequency of normal feces differed significantly among the BA clusters (ANOVA, p = 0.005), where the low-secBA and high-priBA cluster had significantly more-frequent normal feces than high-secBA (Tukey’s post hoc test, p = 0.015; and p = 0.011, respectively).
Differences in intestinal microbiota among BA clusters
Table 3 showed the different intestinal microbiota among BA clusters. The relative abundance of Bacteroides in the high-priBA cluster was significantly lower than the low-, medium-, and high-secBA clusters (Tukey’s post hoc test, p < 0.001; p < 0.001; and p = 0.003, respectively). There was significantly less Clostridium cluster IV in the high-priBA cluster than the high-secBA cluster (Tukey’s post hoc test, p = 0.009). Additionally, there was significantly more Clostridium subcluster XIVa in the high-priBA cluster compared to the low-secBA cluster (Games–Howell’s test, p = 0.029).
Differences in habitual diet among BA clusters
Table 4 indicates the dietary characteristics of the study participants among BA clusters. The intake of animal protein and animal fat differed significantly among BA clusters (ANOVA, p = 0.012 and p = 0.002, respectively), with the low-secBA cluster having the lowest intake. However, the intake of insoluble fiber was significantly higher for high-priBA cluster (ANOVA, p = 0.022).
Principal component analysis
To better interpret the association of defecation status, intestinal microbiota, and habitual diet with fecal BA composition, we performed a PCA on the six variables that showed significant differences among BA clusters (Clostridium cluster IV, Bacteroides, Clostridium subcluster XIVa, normal defecation frequency, insoluble fiber, and animal fat). Animal protein was excluded because its intake was assumed to be associated with the intake of animal fat. The PCA showed that the first principal component (PC1) explained 27.8% of the variance, whereas the second principal component (PC2) explained 22.8%. The major variables of the PC1 were Clostridium cluster IV, Bacteroides, and Clostridium subcluster XIVa; thus, PC1 was considered as a component of the intestinal microbiota (Fig. 4a). Conversely, the major variables of the PC2 were normal defecation frequency, animal fat, and insoluble fiber; hence, PC2 was considered as a component of the defecation and dietary status. Bacteroides was a major component of the PC1, but was also moderately involved in the PC2 (Fig. 4b).
Association of defecation, diet, and intestinal microbiota with fecal bile acid composition in the community-dwelling young participant. Principal component analysis was performed on six variables that showed significant differences among clusters based on the fecal bile acid composition and generated two principal components (PC1 and PC2). a Factor loadings of the PC1. b Factor loadings of the PC2. c PC1 and PC2 plot of the participants according to the bile acid cluster. The center of the ellipse showed the mean values of PC1 and PC2, and the radius showed the standard deviation. PC1 and PC2 were compared among bile acid clusters using a one-way analysis of variance followed by Tukey’s post hoc test. *Tukey’s post hoc test, p < 0.001
The distributions of PC1 and PC2 of the participants were shown in Fig. 4c according to BA clusters. The high-priBA cluster in PC1 was significantly lower than the other three clusters (ANOVA, p < 0.001, Tukey’s post hoc test; high-priBA vs. low-secBA, p < 0.001; high-priBA vs. medium-secBA, p < 0.001; high-priBA vs. high-secBA, p < 0.001). Conversely, the low-secBA cluster in PC2 was significantly higher than the high-secBA cluster and no significantly higher than the medium-secBA cluster (ANOVA, p = 0.002, Tukey’s post hoc test, low-priBA vs. high-secBA, p < 0.001; low-secBA vs. medium-secBA, p = 0.055).
Discussion
To explore the factors affecting the fecal BA composition, the present study investigated a wide range of variables including defecation status, habitual diet, intestinal microbiota, and fecal BA levels. The participants of the study were classified according to their fecal BA composition. In this study, 20.9% of the participants had high fecal BA levels with predominantly priBAs (CA and CDCA) (high-priBA cluster). This cluster was associated with an increased relative abundance of Clostridium subcluster XIVa, increased frequency of normal feces, and decreased relative abundance of Bacteroides and Clostridium cluster IV. Alternatively, high-secBA cluster, which had the same level of total fecal BAs as high-priBA cluster, but with a predominance of secBAs, was associated with an increased animal fat intake, and decreased frequency of normal feces and insoluble fiber intake.
Factors related to high fecal priBA levels
Members of Clostridium subcluster XIVa may assist with production of secBAs. The Clostridium subcluster XIVa contained strains with high BA 7α-dehydroxylating activity [42]. Kakiyama et al. investigated fecal BAs and intestinal microbiota in patients with cirrhosis and healthy participants; they concluded that the relative abundances of Ruminococcaceae and Blautia—members of Clostridium subcluster XIVa—were positively correlated with fecal secBAs levels [43]. Murakami et al. investigated fecal BAs and intestinal microbiota in patients with gastrointestinal diseases and healthy participants and found that the relative abundance of Clostridium subcluster XIVa was positively correlated with the 7α-dehydroxylation marker, the DCA/(DCA + CA) ratio [20].
We found the relative abundance of Clostridium subcluster XIVa to increase in a near-linear fashion among the various secBA level tertiles, although it was highest in high-priBA. A recent study estimated that BA 7α-dehydroxylation comprised only ~ 0.0001% of the total intestinal microbiota and that most Clostridium subcluster XIVa members lacked the bai operon—the gene cluster for the 7α-dehydroxylation [44]. A study that used rats showed that CA feeding increased levels in Clostridium subcluster XIVa members [45]. The proliferation of this cluster member may be further promoted by an increased input of CA into the large intestine [46]. On the other hand, the Clostridium subcluster XIVa contained butyrate-producing bacteria [47]. Increases in the abundance of Clostridium subcluster XIVa in the colons of piglets and mice were observed following dietary fiber supplementation [48, 49].
Zhao et al. examined the BA-related metabolism and metagenome in 290 patients with diarrhea-predominant IBS and 89 healthy participants. Their results indicated UDCA and 7-oxo-DCA may enhance hepatic synthesis and fecal excretion of BAs by attenuation of farnesoid X receptor/fibroblast growth factor 19 signaling [50]. A randomized controlled study in patients with morbid obesity showed that UDCA administration stimulated BA synthesis by reducing circulating fibroblast growth factor 19 and farnesoid X receptor activation [51]. Interestingly, the fecal UDCA and 7-oxo-DCA levels in the high-priBA cluster were significantly higher than levels observed in other clusters. While we don’t know what caused the high fecal priBA levels in the high-priBA cluster, high fiber intake and frequent defecation might have contributed to this finding; additional studies are needed to examine this question. On the other hand, elevated fecal priBA levels were previously observed in individuals with functional bowel disorders and cirrhosis [43, 52].
Factors involved in high fecal secBA levels
We found high fecal secBA levels to be associated with fewer “normal” bowel movements and a higher intake of animal fat. A randomized controlled-feeding trial of healthy young adults showed that consuming a high-fat diet for 6 months increased fecal secBA levels [53]. A short-term dietary intervention study showed that an animal-based diet significantly increased fecal DCA levels and microbial bile salt hydrolases gene expression [24]. Although several studies showed that high-volume intake of fat or animal foods increased BA secretion and was associated with increased fecal secBA levels [54], these studies did not examine defecation status. Conversely, Thomas et al. investigated the relationship between large bowel transit time and the fecal activity of BA metabolizing enzymes in patients with acromegaly [55] and cholesterol cholelithiasis [56]. They found that prolonged colonic transit time was associated with increased activity of the 7α-dehydroxylating enzyme. Hence, the combination of infrequent bowel movements and intake of a high-fat diet may predispose to increased fecal secBA levels. In the present study, the fat intake in the high-secBA cluster was comparable with that of medium-secBA, although participants in the high-secBA cluster tended to have fewer bowel movements.
High-secBA cluster members, who presumably produced more secBAs, were at increased risk of colon cancer [9], cholelithiasis [10], and liver cancer [11]. Conversely, the high-priBA cluster had lower secBAs, but higher priBAs, as well as more-frequent bowel movements and higher dietary fiber intake. Insoluble fibers can hold large amounts of water; increased intake of insoluble fiber promotes intestinal peristalsis and increases fecal volume [57]. Additionally, fibers can bind BAs [58]; thus, increased consumption of dietary insoluble fiber will promote fecal BA excretion, potentially lowering serum cholesterol [59]. Nevertheless, several studies in patients with IBS showed that high fecal priBA levels might be associated with inflammation and poor prognosis [1].
Our study had some limitations. First, its cohort was relatively small and consisted mostly of female. Fecal BA levels were not significantly different between males and females (data not shown), but sex differences in colorectal motility and the prevalence of functional constipation have been reported [60, 61]. Whether fecal bile acid concentrations vary by sex is an interesting question. The female-only dataset analysis also produced results similar to the analysis that included both males and females (Online Resources 5). Second, the T-RFLP method cannot reveal the role of bacterial species and is inferior to next-generation sequencing. Third, BDHQ can assess false intakes through reporting biases. Fourth, because the BSFS scores were self-reported, we cannot exclude the possibility of recall or other individual-level bias. Finally, this was a cross-sectional study, without a control group; hence, causality could not be determined.
Conclusion
High fecal priBA levels were associated with a low relative abundance of Clostridium cluster IV and Bacteroides, a high relative abundance of Clostridium subcluster XIVa, and a high normal defecation frequency. Although the health effects of high fecal priBA or each Clostridium cluster remain unclear, our results provide important insights for regulation of intestinal BA metabolism. Conversely, high levels of cytotoxic secBA were associated with low normal defecation frequency, low insoluble fiber intake, and high animal fat intake. These results indicate that among community-dwelling young adults, secBA production is affected by both dietary and lifestyle-related factors. These results may inform novel strategies for preventing colorectal cancer and cholelithiasis.
Data availability
Deidentified data are available from the corresponding author upon reasonable request.
References
Connors J, Dunn KA, Allott J et al (2020) The relationship between fecal bile acids and microbiome community structure in pediatric Crohn’s disease. ISME J 14:702–713. https://doi.org/10.1038/s41396-019-0560-3
Ridlon JM, Kang D-J, Hylemon PB (2006) Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259. https://doi.org/10.1194/jlr.R500013-JLR200
Batta AK, Salen G, Arora R et al (1990) Side chain conjugation prevents bacterial 7-dehydroxylation of bile acids. J Biol Chem 265:10925–10928. https://doi.org/10.1016/S0021-9258(19)38535-7
Monteiro-Cardoso VF, Corlianò M, Singaraja RR (2021) Bile acids: a communication channel in the gut-brain axis. Neuromolecular Med 23:99–117. https://doi.org/10.1007/s12017-020-08625-z
Hegyi P, Maléth J, Walters JR et al (2018) Guts and gall: bile acids in regulation of intestinal epithelial function in health and disease. Physiol Rev 98:1983–2023. https://doi.org/10.1152/physrev.00054.2017
Chiang JYL, Ferrell JM (2020) Bile acid biology, pathophysiology, and therapeutics. Clin Liver Dis (Hoboken) 15:91–94. https://doi.org/10.1002/cld.861
Fiorucci S, Distrutti E (2015) Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med 21:702–714. https://doi.org/10.1016/j.molmed.2015.09.001
Barrasa JI, Olmo N, Lizarbe MA, Turnay J (2013) Bile acids in the colon, from healthy to cytotoxic molecules. Toxicol In Vitro 27:964–977. https://doi.org/10.1016/j.tiv.2012.12.020
Bernstein H, Bernstein C, Payne CM, Dvorak K (2009) Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol 15:3329–3340. https://doi.org/10.3748/wjg.15.3329
Wang HH, Portincasa P, Wang DQ-H (2008) Molecular pathophysiology and physical chemistry of cholesterol gallstones. Front Biosci 13:401–423. https://doi.org/10.2741/2688
Yoshimoto S, Loo TM, Atarashi K et al (2013) Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499:97–101. https://doi.org/10.1038/nature12347
Kuno T, Hirayama-Kurogi M, Ito S, Ohtsuki S (2018) Reduction in hepatic secondary bile acids caused by short-term antibiotic-induced dysbiosis decreases mouse serum glucose and triglyceride levels. Sci Rep 8:1253. https://doi.org/10.1038/s41598-018-19545-1
Jones ML, Martoni CJ, Prakash S (2012) Cholesterol lowering and inhibition of sterol absorption by Lactobacillus reuteri NCIMB 30242: a randomized controlled trial. Eur J Clin Nutr 66:1234–1241. https://doi.org/10.1038/ejcn.2012.126
Ataie-Jafari A, Larijani B, Alavi Majd H, Tahbaz F (2009) Cholesterol-lowering effect of probiotic yogurt in comparison with ordinary yogurt in mildly to moderately hypercholesterolemic subjects. Ann Nutr Metab 54:22–27. https://doi.org/10.1159/000203284
Ishimwe N, Daliri EB, Lee BH et al (2015) The perspective on cholesterol-lowering mechanisms of probiotics. Mol Nutr Food Res 59:94–105. https://doi.org/10.1002/mnfr.201400548
Ooi L-G, Liong M-T (2010) Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci 11:2499–2522. https://doi.org/10.3390/ijms11062499
Camilleri M (2014) Advances in understanding of bile acid diarrhea. Expert Rev Gastroenterol Hepatol 8:49–61. https://doi.org/10.1586/17474124.2014.851599
Jia B, Park D, Chun BH et al (2021) Diet-related alterations of gut bile salt hydrolases determined using a metagenomic analysis of the human microbiome. Int J Mol Sci 22:3652. https://doi.org/10.3390/ijms22073652
Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023. https://doi.org/10.1038/4441022a
Murakami M, Iwamoto J, Honda A et al (2018) Detection of gut dysbiosis due to reduced Clostridium subcluster XIVa using the fecal or serum bile acid profile. Inflamm Bowel Dis 24:1035–1044. https://doi.org/10.1093/ibd/izy022
Guo P, Zhang K, Ma X, He P (2020) Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol 11:24. https://doi.org/10.1186/s40104-019-0402-1
Venancio VP, Kim H, Sirven MA et al (2018) Polyphenol-rich mango (Mangifera indica L.) ameliorate functional constipation symptoms in humans beyond equivalent amount of fiber. Mol Nutr Food Res 62:e1701034. https://doi.org/10.1002/mnfr.201701034
Alberts DS, Ritenbaugh C, Story JA et al (1996) Randomized, double-blinded, placebo-controlled study of effect of wheat bran fiber and calcium on fecal bile acids in patients with resected adenomatous colon polyps. J Natl Cancer Inst 88:81–92. https://doi.org/10.1093/jnci/88.2.81
David LA, Maurice CF, Carmody RN et al (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. https://doi.org/10.1038/nature12820
Wu GD, Chen J, Hoffmann C et al (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. https://doi.org/10.1126/science.1208344
Marteau P, Cuillerier E, Meance S et al (2002) Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study. Aliment Pharmacol Ther 16:587–593. https://doi.org/10.1046/j.1365-2036.2002.01188.x
Tanabe A, Adachi K, Yamaguchi Y et al (2020) Gut environment and dietary habits in healthy Japanese adults and their association with bowel movement. Digestion 101:706–716. https://doi.org/10.1159/000501961
Hofmann AF, Hagey LR, Krasowski MD (2010) Bile salts of vertebrates: structural variation and possible evolutionary significance. J Lipid Res 51:226–246. https://doi.org/10.1194/jlr.R000042
Kobayashi S, Honda S, Murakami K et al (2012) Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol 22:151–159. https://doi.org/10.2188/jea.JE20110075
Kobayashi S, Yuan X, Sasaki S et al (2019) Relative validity of brief-type self-administered diet history questionnaire among very old Japanese aged 80 years or older. Public Health Nutr 22:212–222. https://doi.org/10.1017/S1368980018002331
O’Donnell LJ, Virjee J, Heaton KW (1990) Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ 300:439–440. https://doi.org/10.1136/bmj.300.6722.439
Longstreth GF, Thompson WG, Chey WD et al (2006) Functional bowel disorders. Gastroenterology 130:1480–1491. https://doi.org/10.1053/j.gastro.2005.11.061
Takahashi S, Tomita J, Nishioka K et al (2014) Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS One 9:e105592. https://doi.org/10.1371/journal.pone.0105592
Nagashima K, Hisada T, Sato M, Mochizuki J (2003) Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl Environ Microbiol 69:1251–1262. https://doi.org/10.1128/AEM.69.2.1251-1262.2003
Nagashima K, Mochizuki J, Hisada T et al (2006) Phylogenetic analysis of 16S ribosomal RNA gene sequences from human fecal microbiota and improved utility of terminal restriction fragment length polymorphism profiling. Biosci Microflora 25:99–107. https://doi.org/10.12938/bifidus.25.99
Collins MD, Lawson PA, Willems A et al (1994) The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol 44:812–826. https://doi.org/10.1099/00207713-44-4-812
Rajilić-Stojanović M, de Vos WM (2014) The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 38:996–1047. https://doi.org/10.1111/1574-6976.12075
Kakiyama G, Muto A, Takei H et al (2014) A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: validation by GC-MS and LC-MS. J Lipid Res 55:978–990. https://doi.org/10.1194/jlr.D047506
(2015) Standard Tables of Food Composition in Japan-2015-(Seventh Revised Edition). Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan
Milligan GW, Cooper MC (1987) Methodology review: clustering methods. Appl Psychol Meas 11:329–354. https://doi.org/10.1177/014662168701100401
Kodinariya TM, Makwana DPR (2013) Review on determining number of cluster in K-means clustering. Int J Adv Res Comput Sci Manag Stud 1:90–95
Kitahara M, Takamine F, Imamura T, Benno Y (2000) Assignment of Eubacterium sp. VPI 12708 and related strains with high bile acid 7alpha-dehydroxylating activity to Clostridium scindens and proposal of Clostridium hylemonae sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 50(3):971–978. https://doi.org/10.1099/00207713-50-3-971
Kakiyama G, Pandak WM, Gillevet PM et al (2013) Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol 58:949–955. https://doi.org/10.1016/j.jhep.2013.01.003
Solbach P, Chhatwal P, Woltemate S et al (2018) BaiCD gene cluster abundance is negatively correlated with Clostridium difficile infection. PLoS One 13:e0196977. https://doi.org/10.1371/journal.pone.0196977
Islam KBMS, Fukiya S, Hagio M et al (2011) Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141:1773–1781. https://doi.org/10.1053/j.gastro.2011.07.046
Ridlon JM, Alves JM, Hylemon PB, Bajaj JS (2013) Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes 4:382–387. https://doi.org/10.4161/gmic.25723
Tamanai-Shacoori Z, Smida I, Bousarghin L et al (2017) Roseburia spp.: a marker of health? Future Microbiol 12:157–170. https://doi.org/10.2217/fmb-2016-0130
Mu C, Zhang L, He X et al (2017) Dietary fibres modulate the composition and activity of butyrate-producing bacteria in the large intestine of suckling piglets. Antonie Van Leeuwenhoek 110:687–696. https://doi.org/10.1007/s10482-017-0836-4
Lange K, Hugenholtz F, Jonathan MC et al (2015) Comparison of the effects of five dietary fibers on mucosal transcriptional profiles, and luminal microbiota composition and SCFA concentrations in murine colon. Mol Nutr Food Res 59:1590–1602. https://doi.org/10.1002/mnfr.201400597
Zhao L, Yang W, Chen Y et al (2020) A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J Clin Invest 130:438–450. https://doi.org/10.1172/JCI130976
Mueller M, Thorell A, Claudel T et al (2015) Ursodeoxycholic acid exerts farnesoid X receptor-antagonistic effects on bile acid and lipid metabolism in morbid obesity. J Hepatol 62:1398–1404. https://doi.org/10.1016/j.jhep.2014.12.034
Vijayvargiya P, Gonzalez Izundegui D, Calderon G et al (2020) Fecal bile acid testing in assessing patients with chronic unexplained diarrhea: implications for healthcare utilization. Am J Gastroenterol 115:1094–1102. https://doi.org/10.14309/ajg.0000000000000637
Wan Y, Yuan J, Li J et al (2020) Unconjugated and secondary bile acid profiles in response to higher-fat, lower-carbohydrate diet and associated with related gut microbiota: a 6-month randomized controlled-feeding trial. Clin Nutr 39:395–404. https://doi.org/10.1016/j.clnu.2019.02.037
Thorning TK, Raziani F, Bendsen NT et al (2015) Diets with high-fat cheese, high-fat meat, or carbohydrate on cardiovascular risk markers in overweight postmenopausal women: a randomized crossover trial. Am J Clin Nutr 102:573–581. https://doi.org/10.3945/ajcn.115.109116
Thomas LA, Veysey MJ, Murphy GM et al (2005) Octreotide induced prolongation of colonic transit increases faecal anaerobic bacteria, bile acid metabolising enzymes, and serum deoxycholic acid in patients with acromegaly. Gut 54:630–635. https://doi.org/10.1136/gut.2003.028431
Thomas LA, Veysey MJ, Bathgate T et al (2000) Mechanism for the transit-induced increase in colonic deoxycholic acid formation in cholesterol cholelithiasis. Gastroenterology 119:806–815. https://doi.org/10.1053/gast.2000.16495
Gemen R, de Vries JF, Slavin JL (2011) Relationship between molecular structure of cereal dietary fiber and health effects: focus on glucose/insulin response and gut health. Nutr Rev 69:22–33. https://doi.org/10.1111/j.1753-4887.2010.00357.x
Singh J, Metrani R, Shivanagoudra SR et al (2019) Review on bile acids: effects of the gut microbiome, interactions with dietary fiber, and alterations in the bioaccessibility of bioactive compounds. J Agric Food Chem 67:9124–9138. https://doi.org/10.1021/acs.jafc.8b07306
Lia A, Hallmans G, Sandberg AS et al (1995) Oat beta-glucan increases bile acid excretion and a fiber-rich barley fraction increases cholesterol excretion in ileostomy subjects. Am J Clin Nutr 62:1245–1251. https://doi.org/10.1093/ajcn/62.6.1245
Horii K, Ehara Y, Shiina T et al (2021) Sexually dimorphic response of colorectal motility to noxious stimuli in the colorectum in rats. J Physiol 599:1421–1437. https://doi.org/10.1113/JP279942
Lim YJ, Rosita J, Chieng JY, Hazizi AS (2016) The prevalence and symptoms characteristic of functional constipation using Rome III diagnostic criteria among tertiary education students. PLoS One 11:e0167243. https://doi.org/10.1371/journal.pone.0167243
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
This work was supported by the Grant-in-Aid for Young Scientists (grant number: 19K20148) from the Japan Society for the Promotion of Science (YS grantee). The funding source had no involvement in study execution or manuscript preparation.
Author information
Authors and Affiliations
Contributions
YS: term, conceptualization, methodology, formal analysis, investigation, resources, writing-original draft, writing-editing, visualization, supervision, project administration, funding acquisition. TS: term, conceptualization, methodology, investigation, resources, writing-review and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the participant matter or materials discussed in this manuscript. The authors declare that they have no conflicts of interest to declare.
Ethical approval
The study protocol was approved by the Ethics Committee of the Yamagata Prefectural Yonezawa University of Nutrition Sciences (Approval No. 2019–9).
Informed consent
All study participants provided written informed consent for participation and publication of their de-identified data.
Additional information
Yosuke Saito was transferred to Hiroshima International University during this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saito, Y., Sagae, T. Defecation status, intestinal microbiota, and habitual diet are associated with the fecal bile acid composition: a cross-sectional study in community-dwelling young participants. Eur J Nutr 62, 2015–2026 (2023). https://doi.org/10.1007/s00394-023-03126-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03126-8