Abstract
Purpose
New dietary proteins are currently introduced to replace traditional animal protein sources. However, not much is known about their bioaccessibility and ability to stimulate muscle protein synthesis compared to the traditional protein sources. We aimed to compare effects of ingesting a protein bolus (0.25 g/kg fat free mass) of either cricket (insect), pea, or whey protein on circulating amino acid levels and activation of the mTORC1 signaling pathway in the skeletal muscle at rest and after exercise.
Methods
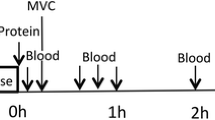
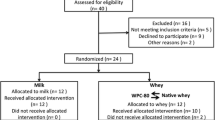
In a randomized parallel controlled trial, young males (n = 50) performed a one-legged resistance exercise followed by ingestion of one of the three protein sources. Blood samples were collected before and in the following 4 h after exercise. Muscle biopsies were obtained at baseline and after 3 h from the non-exercised and exercised leg.
Results
Analysis of blood serum showed a significantly higher concentration of amino acids after ingestion of whey protein compared to cricket and pea protein. No difference between protein sources in activation of the mTORC1 signaling pathway was observed either at rest or after exercise.
Conclusion
Amino acid blood concentration after protein ingestion was higher for whey than pea and cricket protein, whereas activation of mTORC1 signaling pathway at rest and after exercise did not differ between protein sources.
Trial registration number
Clinicaltrials.org ID NCT04633694.




Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ (2012) Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 96(6):1454–1464. https://doi.org/10.3945/ajcn.112.037556
Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, Aragon AA, Devries MC, Banfield L, Krieger JW, Phillips SM (2018) A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med 52(6):376–384. https://doi.org/10.1136/bjsports-2017-097608
Moore DR (2021) Protein requirements for master athletes: just older versions of their younger selves. Sports Med. https://doi.org/10.1007/s40279-021-01510-0
Jäger R, Kerksick CM, Campbell BI, Cribb PJ, Wells SD, Skwiat TM, Purpura M, Ziegenfuss TN, Ferrando AA, Arent SM, Smith-Ryan AE, Stout JR, Arciero PJ, Ormsbee MJ, Taylor LW, Wilborn CD, Kalman DS, Kreider RB, Willoughby DS, Hoffman JR, Krzykowski JL, Antonio J (2017) International society of sports nutrition position stand: protein and exercise. J Int Soc Sports Nutr 14(1):20. https://doi.org/10.1186/s12970-017-0177-8
van Vliet S, Burd NA, van Loon LJ (2015) The skeletal muscle anabolic response to plant- versus animal-based protein consumption. J Nutr 145(9):1981–1991. https://doi.org/10.3945/jn.114.204305
Phillips SM (2016) The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr Metab 13:64. https://doi.org/10.1186/s12986-016-0124-8
Burd NA, Beals JW, Martinez IG, Salvador AF, Skinner SK (2019) Food-first approach to enhance the regulation of post-exercise skeletal muscle protein synthesis and remodeling. Sports Med 49(Suppl 1):59–68. https://doi.org/10.1007/s40279-018-1009-y
Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ (2010) Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 38(5):1533–1539. https://doi.org/10.1007/s00726-009-0377-x
Tang JE, Phillips SM (2009) Maximizing muscle protein anabolism: the role of protein quality. Curr Opin Clin Nutr Metab Care 12(1):66–71. https://doi.org/10.1097/MCO.0b013e32831cef75
Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM (2009) Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 107(3):987–992. https://doi.org/10.1152/japplphysiol.00076.2009
Devries MC, Phillips SM (2015) Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci 80(Suppl 1):A8-a15. https://doi.org/10.1111/1750-3841.12802
Sranacharoenpong K, Soret S, Harwatt H, Wien M, Sabate J (2015) The environmental cost of protein food choices. Public Health Nutr 18(11):2067–2073. https://doi.org/10.1017/s1368980014002377
Gorissen SHM, Crombag JJR, Senden JMG, Waterval WAH, Bierau J, Verdijk LB, van Loon LJC (2018) Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 50(12):1685–1695. https://doi.org/10.1007/s00726-018-2640-5
Scholz-Ahrens KE, Ahrens F, Barth CA (2020) Nutritional and health attributes of milk and milk imitations. Eur J Nutr 59(1):19–34. https://doi.org/10.1007/s00394-019-01936-3
Churchward-Venne TA, Pinckaers PJM, van Loon JJA, van Loon LJC (2017) Consideration of insects as a source of dietary protein for human consumption. Nutr Rev 75(12):1035–1045. https://doi.org/10.1093/nutrit/nux057
Rumpold BA, Schluter OK (2013) Nutritional composition and safety aspects of edible insects. Mol Nutr Food Res 57(5):802–823. https://doi.org/10.1002/mnfr.201200735
Hermans WJH, Senden JM, Churchward-Venne TA, Paulussen KJM, Fuchs CJ, Smeets JSJ, van Loon JJA, Verdijk LB, van Loon LJC (2021) Insects are a viable protein source for human consumption: from insect protein digestion to postprandial muscle protein synthesis in vivo in humans: a double-blind randomized trial. Am J Clin Nutr. https://doi.org/10.1093/ajcn/nqab115
Vangsoe MT, Joergensen MS, Heckmann LL, Hansen M (2018) Effects of insect protein supplementation during resistance training on changes in muscle mass and strength in young men. Nutrients 10(3):335. https://doi.org/10.3390/nu10030335
Vangsoe MT, Thogersen R, Bertram HC, Heckmann LL, Hansen M (2018) Ingestion of insect protein isolate enhances blood amino acid concentrations similar to soy protein in a human trial. Nutrients 10(10):1357. https://doi.org/10.3390/nu10101357
Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM (2015) Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 70(1):57–62. https://doi.org/10.1093/gerona/glu103
Moro T, Brightwell CR, Velarde B, Fry CS, Nakayama K, Sanbongi C, Volpi E, Rasmussen BB (2019) Whey protein hydrolysate increases amino acid uptake, mTORC1 signaling, and protein synthesis in skeletal muscle of healthy young men in a randomized crossover trial. J Nutr 149(7):1149–1158. https://doi.org/10.1093/jn/nxz053
Phillips SM, Chevalier S, Leidy HJ (2016) Protein “requirements” beyond the RDA: implications for optimizing health. Appl Physiol Nutr Metab 41(5):565–572. https://doi.org/10.1139/apnm-2015-0550%M26960445
Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR (2004) Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab 286(3):E321-328. https://doi.org/10.1152/ajpendo.00368.2003
Tipton KD, Gurkin BE, Matin S, Wolfe RR (1999) Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem 10(2):89–95. https://doi.org/10.1016/s0955-2863(98)00087-4
Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR (2003) Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78(2):250–258. https://doi.org/10.1093/ajcn/78.2.250
Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS (2005) Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr 135(3):376–382. https://doi.org/10.1093/jn/135.3.376
Meyer-Rochow VB, Gahukar RT, Ghosh S, Jung C (2021) Chemical composition, nutrient quality and acceptability of edible insects are affected by species, developmental stage, gender, diet, and processing method. Foods 10(5):1036
Wang N, Hatcher DW, Gawalko EJ (2008) Effect of variety and processing on nutrients and certain anti-nutrients in field peas (Pisum sativum). Food Chem 111(1):132–138. https://doi.org/10.1016/j.foodchem.2008.03.047
Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB (2007) Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol 582(Pt 2):813–823. https://doi.org/10.1113/jphysiol.2007.134593
D’Hulst G, Masschelein E, De Bock K (2021) Dampened muscle mTORC1 response following ingestion of high-quality plant-based protein and insect protein compared to whey. Nutrients 13(5):1396
Weijzen MEG, van Gassel RJJ, Kouw IWK, Trommelen J, Gorissen SHM, van Kranenburg J, Goessens JPB, van de Poll MCG, Verdijk LB, van Loon LJC (2021) Ingestion of free amino acids compared with an equivalent amount of intact protein results in more rapid amino acid absorption and greater postprandial plasma amino acid availability without affecting muscle protein synthesis rates in young adults in a double-blind randomized trial. J Nutr. https://doi.org/10.1093/jn/nxab305
Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB (2008) Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 294(2):E392-400. https://doi.org/10.1152/ajpendo.00582.2007
Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB (2008) Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 104(5):1452–1461. https://doi.org/10.1152/japplphysiol.00021.2008
Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, Rasmussen BB (2010) Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol 299(2):R533-540. https://doi.org/10.1152/ajpregu.00077.2010
Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR (2000) An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol 88(2):386–392. https://doi.org/10.1152/jappl.2000.88.2.386
Børsheim E, Tipton KD, Wolf SE, Wolfe RR (2002) Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab 283(4):E648-657. https://doi.org/10.1152/ajpendo.00466.2001
van Loon LJ, Saris WH, Verhagen H, Wagenmakers AJ (2000) Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am J Clin Nutr 72(1):96–105. https://doi.org/10.1093/ajcn/72.1.96
Bell JA, Fujita S, Volpi E, Cadenas JG, Rasmussen BB (2005) Short-term insulin and nutritional energy provision do not stimulate muscle protein synthesis if blood amino acid availability decreases. Am J Physiol-Endocrinol Metab 289(6):E999–E1006. https://doi.org/10.1152/ajpendo.00170.2005
Bennet WM, Connacher AA, Scrimgeour CM, Jung RT, Rennie MJ (1990) Euglycemic hyperinsulinemia augments amino acid uptake by human leg tissues during hyperaminoacidemia. Am J Physiol-Endocrinol Metab 259(2):E185–E194. https://doi.org/10.1152/ajpendo.1990.259.2.E185
Biolo G, Declan Fleming RY, Wolfe RR (1995) Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Investig 95(2):811–819. https://doi.org/10.1172/JCI117731
Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E (2006) Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol-Endocrinol Metab 291(4):E745–E754. https://doi.org/10.1152/ajpendo.00271.2005
Yoon MS (2016) The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 8(7):405. https://doi.org/10.3390/nu8070405
Gelfand RA, Barrett EJ (1987) Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Investig 80(1):1–6. https://doi.org/10.1172/JCI113033
Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ (2010) Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92(5):1080–1088. https://doi.org/10.3945/ajcn.2010.29819
Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB (2009) Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 106(4):1374–1384. https://doi.org/10.1152/japplphysiol.91397.2008
Yoon M-S (2017) mTOR as a key regulator in maintaining skeletal muscle mass. Front Physiol. https://doi.org/10.3389/fphys.2017.00788
Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB (2011) Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1(1):11. https://doi.org/10.1186/2044-5040-1-11
Dalgaard LB, Dalgas U, Andersen JL, Rossen NB, Møller AB, Stødkilde-Jørgensen H, Jørgensen JO, Kovanen V, Couppé C, Langberg H, Kjær M, Hansen M (2019) Influence of oral contraceptive use on adaptations to resistance training. Front Physiol 10:824. https://doi.org/10.3389/fphys.2019.00824
Dam TV, Dalgaard LB, Ringgaard S, Johansen FT, Bisgaard Bengtsen M, Mose M, Lauritsen KM, Ørtenblad N, Gravholt CH, Hansen M (2020) transdermal estrogen therapy improves gains in skeletal muscle mass after 12 weeks of resistance training in early postmenopausal women. Front Physiol 11:596130. https://doi.org/10.3389/fphys.2020.596130
Hansen M, Skovgaard D, Reitelseder S, Holm L, Langbjerg H, Kjaer M (2012) Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci 67(10):1005–1013. https://doi.org/10.1093/gerona/gls007
Gorissen SHM, Burd NA, Kramer IF, van Kranenburg J, Gijsen AP, Rooyackers O, van Loon LJC (2017) Co-ingesting milk fat with micellar casein does not affect postprandial protein handling in healthy older men. Clin Nutr 36(2):429–437. https://doi.org/10.1016/j.clnu.2015.12.011
Church DD, Hirsch KR, Park S, Kim I-Y, Gwin JA, Pasiakos SM, Wolfe RR, Ferrando AA (2020) Essential amino acids and protein synthesis: insights into maximizing the muscle and whole-body response to feeding. Nutrients 12(12):3717
Walker DK, Dickinson JM, Timmerman KL, Drummond MJ, Reidy PT, Fry CS, Gundermann DM, Rasmussen BB (2011) Exercise, amino acids, and aging in the control of human muscle protein synthesis. Med Sci Sports Exerc 43(12):2249–2258. https://doi.org/10.1249/MSS.0b013e318223b037
Acknowledgements
Data were generated though accessing research infrastructure at Aarhus University, including FOODHAY (Food and Health Open Innovation Laboratory, Danish Roadmap for Research Infrastructure). The authors thank Janni Mosgaard Jensen and Gitte Kaiser Hartvigsen for technical support during the experimental days and for western blotting analysis.
Funding
This research was supported by Fonden til Lægevidenskabens Fremme, Copenhagen, Denmark and Beckett-fonden, Denmark (19–2-4811). The present study was part of Sofie Kaas Lanng’s PhD project, which was funded by CiFOOD, Centre for Innovative Food Research, Aarhus University and HCB’s Eliteforsk grant (6161-00016B).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lanng, S.K., Oxfeldt, M., Pedersen, S.S. et al. Influence of protein source (cricket, pea, whey) on amino acid bioavailability and activation of the mTORC1 signaling pathway after resistance exercise in healthy young males. Eur J Nutr 62, 1295–1308 (2023). https://doi.org/10.1007/s00394-022-03071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03071-y