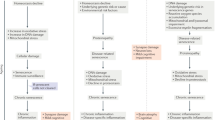
Abstract
Introduction
Hayflick and Moorhead first demonstrated cell senescence as the irreversible growth arrest of cells after prolonged cultivation. Telomere shortening and oxidative stress are the fundamental mechanisms that drive cell senescence. Increasing studies have shown that TMAO is closely associated with cellular aging and age-related diseases. An emerging body of evidence from animal models, especially mice, has identified that TMAO contributes to senescence from multiple pathways and appears to accelerate many neurodegenerative disorders such as Alzheimer’s disease and Parkinson’s disease. However, the specific mechanism of how TMAO speeds aging is still not completely clear.
Material and methods
In this review, we summarize some key findings in TMAO, cell senescence, and age-related diseases. We focused particular attention on the potential mechanisms for clinical transformation to find ways to interfere with the aging process.
Conclusion
TMAO can accelerate cell senescence by causing mitochondrial damage, superoxide formation, and promoting the generation of pro-inflammatory factors.



Similar content being viewed by others
Availability of data and materials
There are no original data in our manuscript.
Abbreviations
- 1H-NMR:
-
Proton nuclear magnetic resonance spectrometry
- Aβ:
-
β-Amyloid peptide
- AD:
-
Alzheimer’s disease
- AS:
-
Atherosclerosis
- βCTF:
-
β-Secretase C-terminal fragment
- CHD:
-
Chow diet
- CSF:
-
Cerebrospinal fluid
- cntA/B:
-
Carnitine monooxygenase
- cutC/D:
-
Choline-TMA lyase
- DAMPs:
-
Damage-associated molecular patterns
- DBM:
-
3,3-Dimethyl-1-butanol
- DDR:
-
DNA damage response
- DMB:
-
3,3-Dimethyl-1-butanol
- DNA-SCARS:
-
DNA segments with chromatin alterations reinforcing senescence
- EC:
-
Endothelial cell
- ECM:
-
Extracellular matrix
- ER:
-
Endoplasmic reticulum
- FMO3:
-
Flavin-containing monooxygenase 3
- GBB:
-
γ-Butyrobetaine
- hCETP:
-
Human cholesterol ester transfer protein
- HFD:
-
High-fat diet
- HFHC:
-
High fat and high cholesterol
- HPLC/DMS-MS/MS:
-
Liquid chromatography/differential ion mobility spectrometry tandem mass spectrometry
- HUVEC:
-
Human umbilical vein endothelial cells
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- LTP:
-
Long-term potentiation
- MAFLD:
-
Metabolic dysfunction-associated fatty liver disease
- MMP:
-
Matrix metalloproteinases
- MMP2:
-
Matrix metalloproteinase 2
- MMP9:
-
Matrix metalloproteinase 9
- MS:
-
Multiple sclerosis
- NFL:
-
Neurofilament protein
- NFTs:
-
Neurofibrillary tangles
- NMDAR1:
-
N-methyl-d-asperate receptor 1
- OIS:
-
Oncogene-induced senescence
- Ops:
-
Oligodendrocyte progenitors
- PD:
-
Parkinson’s disease
- PDD:
-
Parkinson’s disease dementia
- PSD-95:
-
Postsynaptic density-95 kDa
- ROS:
-
Oxygen species
- SAMP8:
-
Senescence-accelerated prone mouse strain 8
- SAMR1:
-
Senescence-accelerated mouse resistant 1
- SASP:
-
Senescence-associated secretory phenotype
- SHRs:
-
Spontaneously hypertensive rats
- SIRT1:
-
Sirtuin 1
- SYN:
-
Synaptophysin
- TMA:
-
Trimethylamine
- TMAO:
-
Trimethylamine N-oxide
- TNF-α:
-
Tumor necrosis factor-α
- VSMC:
-
Vascular smooth muscle cell
- WKY:
-
Wistar-Kyoto
References
Jang JY, Blum A, Liu J, Finkel T (2018) The role of mitochondria in aging. J Clin Investig 128(9):3662–3670. https://doi.org/10.1172/jci120842
Kirkwood TB (2005) Understanding the odd science of aging. Cell 120(4):437–447. https://doi.org/10.1016/j.cell.2005.01.027
Kirkland JL, Tchkonia T (2017) Cellular senescence: a translational perspective. EBioMedicine 21:21–28. https://doi.org/10.1016/j.ebiom.2017.04.013
Pignolo RJ, Passos JF, Khosla S, Tchkonia T, Kirkland JL (2020) Reducing senescent cell burden in aging and disease. Trends Mol Med 26(7):630–638. https://doi.org/10.1016/j.molmed.2020.03.005
Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC, Mitchell SC, Holmes E, McCarthy MI, Scott J, Gauguier D, Nicholson JK (2006) Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA 103(33):12511–12516. https://doi.org/10.1073/pnas.0601056103
Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV (2008) Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 455(7216):1109–1113. https://doi.org/10.1038/nature07336
Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 101(44):15718–15723. https://doi.org/10.1073/pnas.0407076101
Sergeev IN, Aljutaily T, Walton G, Huarte E (2020) Effects of synbiotic supplement on human gut microbiota, body composition and weight loss in obesity. Nutrients 12:1. https://doi.org/10.3390/nu12010222
Quigley EMM (2017) Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep 17(12):94. https://doi.org/10.1007/s11910-017-0802-6
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK (2016) Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 167(6):1469-1480.e1412. https://doi.org/10.1016/j.cell.2016.11.018
Wang X, Sun G, Feng T, Zhang J, Huang X, Wang T, Xie Z, Chu X, Yang J, Wang H, Chang S, Gong Y, Ruan L, Zhang G, Yan S, Lian W, Du C, Yang D, Zhang Q, Lin F, Liu J, Zhang H, Ge C, Xiao S, Ding J, Geng M (2019) Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res 29(10):787–803. https://doi.org/10.1038/s41422-019-0216-x
Chen K, Zheng X, Feng M, Li D, Zhang H (2017) Gut microbiota-dependent metabolite trimethylamine N-oxide contributes to cardiac dysfunction in western diet-induced obese mice. Front Physiol 8:139. https://doi.org/10.3389/fphys.2017.00139
Rohrmann S, Linseisen J, Allenspach M, von Eckardstein A, Müller D (2016) plasma concentrations of trimethylamine-N-oxide are directly associated with dairy food consumption and low-grade inflammation in a german adult population. J Nutr 146(2):283–289. https://doi.org/10.3945/jn.115.220103
Pascal MC, Burini JF, Chippaux M (1984) Regulation of the trimethylamine N-oxide (TMAO) reductase in escherichia coli: analysis of tor::mud1 operon fusion. Mol Gen Genet MGG 195(1–2):351–355. https://doi.org/10.1007/bf00332770
Craciun S, Marks JA, Balskus EP (2014) Characterization of choline trimethylamine-lyase expands the chemistry of glycyl radical enzymes. ACS Chem Biol 9(7):1408–1413. https://doi.org/10.1021/cb500113p
Zhu Y, Jameson E, Crosatti M, Schäfer H, Rajakumar K, Bugg TD, Chen Y (2014) Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci USA 111(11):4268–4273. https://doi.org/10.1073/pnas.1316569111
Andreesen JR (1994) Glycine metabolism in anaerobes. Antonie Van Leeuwenhoek 66(1–3):223–237. https://doi.org/10.1007/bf00871641
Ke Y, Li D, Zhao M, Liu C, Liu J, Zeng A, Shi X, Cheng S, Pan B, Zheng L, Hong H (2018) Gut flora-dependent metabolite trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radical Biol Med 116:88–100. https://doi.org/10.1016/j.freeradbiomed.2018.01.007
Flood JF, Morley JE (1998) Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev 22(1):1–20. https://doi.org/10.1016/s0149-7634(96)00063-2
Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, Richey JJ, Zigler MC, Neilson AP, Davy KP, Seals DR (2020) Trimethylamine-N-oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension 76(1):101–112. https://doi.org/10.1161/hypertensionaha.120.14759
Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A (2019) Cellular senescence: aging, cancer, and injury. Physiol Rev 99(2):1047–1078. https://doi.org/10.1152/physrev.00020.2018
Hernandez-Segura A, Nehme J, Demaria M (2018) Hallmarks of cellular senescence. Trends Cell Biol 28(6):436–453. https://doi.org/10.1016/j.tcb.2018.02.001
Casella G, Tsitsipatis D, Abdelmohsen K, Gorospe M (2019) mRNA methylation in cell senescence. Wiley Interdiscip Rev RNA 10(6):e1547. https://doi.org/10.1002/wrna.1547
He S, Sharpless NE (2017) Senescence in health and disease. Cell 169(6):1000–1011. https://doi.org/10.1016/j.cell.2017.05.015
Adams PD (2009) Healing and hurting: molecular mechanisms, functions, and pathologies of cellular senescence. Mol Cell 36(1):2–14. https://doi.org/10.1016/j.molcel.2009.09.021
Bernadotte A, Mikhelson VM, Spivak IM (2016) Markers of cellular senescence telomere shortening as a marker of cellular senescence. Aging 8(1):3–11
Rezzani R, Nardo L, Favero G, Peroni M, Rodella LF (2014) Thymus and aging: morphological, radiological, and functional overview. Age (Dordr) 36(1):313–351. https://doi.org/10.1007/s11357-013-9564-5
Childs BG, Durik M, Baker DJ, van Deursen JM (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21(12):1424–1435. https://doi.org/10.1038/nm.4000
Salminen A, Kauppinen A, Kaarniranta K (2012) Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell Signal 24(4):835–845. https://doi.org/10.1016/j.cellsig.2011.12.006
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6(12):2853–2868. https://doi.org/10.1371/journal.pbio.0060301
Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, Holtz A, Shah S, Sharma V, Ferrucci L, Campisi J, Schilling B (2020) A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol 18(1):e3000599. https://doi.org/10.1371/journal.pbio.3000599
Wiley CD, Liu S, Limbad C, Zawadzka AM, Beck J, Demaria M, Artwood R, Alimirah F, Lopez-Dominguez JA, Kuehnemann C, Danielson SR, Basisty N, Kasler HG, Oron TR, Desprez PY, Mooney SD, Gibson BW, Schilling B, Campisi J, Kapahi P (2019) SILAC analysis reveals increased secretion of hemostasis-related factors by senescent cells. Cell Rep 28(13):3329-3337.e5. https://doi.org/10.1016/j.celrep.2019.08.049
Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J (2013) p53-Dependent release of alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol 201(4):613–629. https://doi.org/10.1083/jcb.201206006
Birch J, Gil J (2020) Senescence and the SASP: many therapeutic avenues. Genes Dev 34(23–24):1565–1576. https://doi.org/10.1101/gad.343129.120
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621. https://doi.org/10.1016/0014-4827(61)90192-6
Xu Z, Teixeira MT (2019) The many types of heterogeneity in replicative senescence. Yeast 36(11):637–648. https://doi.org/10.1002/yea.3433
Nakayama Y, Yamaguchi N (2013) Role of cyclin B1 levels in DNA damage and DNA damage-induced senescence. Int Rev Cell Mol Biol 305:303–337. https://doi.org/10.1016/b978-0-12-407695-2.00007-x
Gorgoulis VG, Halazonetis TD (2010) Oncogene-induced senescence: the bright and dark side of the response. Curr Opin Cell Biol 22(6):816–827. https://doi.org/10.1016/j.ceb.2010.07.013
Saretzki G (2010) Cellular senescence in the development and treatment of cancer. Curr Pharm Des 16(1):79–100. https://doi.org/10.2174/138161210789941874
Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS (2012) Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med 18(9):1359–1368. https://doi.org/10.1038/nm.2890
Gilbert LA, Hemann MT (2010) DNA damage-mediated induction of a chemoresistant niche. Cell 143(3):355–366. https://doi.org/10.1016/j.cell.2010.09.043
Liu D, Hornsby PJ (2007) Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Can Res 67(7):3117–3126. https://doi.org/10.1158/0008-5472.Can-06-3452
Coppé JP, Kauser K, Campisi J, Beauséjour CM (2006) Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J Biol Chem 281(40):29568–29574. https://doi.org/10.1074/jbc.M603307200
Kritsilis M, S VR, Koutsoudaki PN, Evangelou K, Gorgoulis VG, Papadopoulos D (2018) Ageing, cellular senescence and neurodegenerative disease. Int J Mol Sci 19:10. https://doi.org/10.3390/ijms19102937
Long JM, Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179(2):312–339. https://doi.org/10.1016/j.cell.2019.09.001
Goedert M, Klug A, Crowther RA (2006) Tau protein, the paired helical filament and Alzheimer’s disease. J Alzheimer’s Dis 9(3 Suppl):195–207. https://doi.org/10.3233/jad-2006-9s323
DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27(5):457–464. https://doi.org/10.1002/ana.410270502
Rajmohan R, Reddy PH (2017) Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J Alzheimer’s Dis 57(4):975–999. https://doi.org/10.3233/jad-160612
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14(4):388–405. https://doi.org/10.1016/s1474-4422(15)70016-5
Han X, Zhang T, Liu H, Mi Y, Gou X (2020) Astrocyte senescence and Alzheimer’s disease: a review. Front Aging Neurosci 12:148. https://doi.org/10.3389/fnagi.2020.00148
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479(7372):232–236. https://doi.org/10.1038/nature10600
Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H (2011) Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci 34(1):3–11. https://doi.org/10.1111/j.1460-9568.2011.07738.x
Bhat R, Crowe EP, Bitto A, Moh M, Katsetos CD, Garcia FU, Johnson FB, Trojanowski JQ, Sell C, Torres C (2012) Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE 7(9):e45069. https://doi.org/10.1371/journal.pone.0045069
Boccardi V, Pelini L, Ercolani S, Ruggiero C, Mecocci P (2015) From cellular senescence to Alzheimer’s disease: the role of telomere shortening. Ageing Res Rev 22:1–8. https://doi.org/10.1016/j.arr.2015.04.003
Zhang P, Kishimoto Y, Grammatikakis I, Gottimukkala K, Cutler RG, Zhang S, Abdelmohsen K, Bohr VA, Misra Sen J, Gorospe M, Mattson MP (2019) Senolytic therapy alleviates Aβ-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nat Neurosci 22(5):719–728. https://doi.org/10.1038/s41593-019-0372-9
He N, Jin WL, Lok KH, Wang Y, Yin M, Wang ZJ (2013) Amyloid-β(1–42) oligomer accelerates senescence in adult hippocampal neural stem/progenitor cells via formylpeptide receptor 2. Cell Death Dis 4(11):e924. https://doi.org/10.1038/cddis.2013.437
Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, Baker DJ (2018) Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562(7728):578–582. https://doi.org/10.1038/s41586-018-0543-y
Chinta SJ, Woods G, Demaria M, Rane A, Zou Y, McQuade A, Rajagopalan S, Limbad C, Madden DT, Campisi J, Andersen JK (2018) Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Rep 22(4):930–940. https://doi.org/10.1016/j.celrep.2017.12.092
Ihara Y, Morishima-Kawashima M, Nixon R (2012) The ubiquitin-proteasome system and the autophagic-lysosomal system in Alzheimer disease. Cold Spring Harb Perspect Med 2:8. https://doi.org/10.1101/cshperspect.a006361
Blum-Degen D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P (1995) Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202(1–2):17–20. https://doi.org/10.1016/0304-3940(95)12192-7
Dursun E, Gezen-Ak D, Hanağası H, Bilgiç B, Lohmann E, Ertan S, Atasoy İL, Alaylıoğlu M, Araz ÖS, Önal B, Gündüz A, Apaydın H, Kızıltan G, Ulutin T, Gürvit H, Yılmazer S (2015) The interleukin 1 alpha, interleukin 1 beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s disease, mild cognitive impairment or Parkinson’s disease. J Neuroimmunol 283:50–57. https://doi.org/10.1016/j.jneuroim.2015.04.014
Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T (1994) Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett 180(2):147–150. https://doi.org/10.1016/0304-3940(94)90508-8
Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O (2012) Non-motor symptoms in patients with Parkinson’s disease–correlations with inflammatory cytokines in serum. PLoS ONE 7(10):e47387. https://doi.org/10.1371/journal.pone.0047387
Scalzo P, Kümmer A, Cardoso F, Teixeira AL (2010) Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neurosci Lett 468(1):56–58. https://doi.org/10.1016/j.neulet.2009.10.062
Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T (1994) Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett 165(1–2):208–210. https://doi.org/10.1016/0304-3940(94)90746-3
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372(9648):1502–1517. https://doi.org/10.1016/s0140-6736(08)61620-7
Mahad D, Lassmann H, Turnbull D (2008) Review: mitochondria and disease progression in multiple sclerosis. Neuropathol Appl Neurobiol 34(6):577–589. https://doi.org/10.1111/j.1365-2990.2008.00987.x
Gilgun-Sherki Y, Melamed E, Offen D (2004) The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol 251(3):261–268. https://doi.org/10.1007/s00415-004-0348-9
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, Augustine AD, McElhaney JE, Kohanski R, Sierra F (2013) The role of inflammation in age-related disease. Aging 5(1):84–93. https://doi.org/10.18632/aging.100531
Nicaise AM, Wagstaff LJ, Willis CM, Paisie C, Chandok H, Robson P, Fossati V, Williams A, Crocker SJ (2019) Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc Natl Acad Sci USA 116(18):9030–9039. https://doi.org/10.1073/pnas.1818348116
Franklin RJ, Goldman SA (2015) Glia disease and repair-remyelination. Cold Spring Harb Perspect Biol 7(7):a020594. https://doi.org/10.1101/cshperspect.a020594
Mei F, Lehmann Horn K, Shen YA, Rankin KA, Stebbins KJ, Lorrain DS, Pekarek K, S AS, Xiao L, Teuscher C, von Büdingen HC, Wess J, Lawrence JJ, Green AJ, Fancy SP, Zamvil SS, Chan JR (2016) Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife. https://doi.org/10.7554/eLife.1824
Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJ, Casaccia-Bonnefil P (2008) Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci 11(9):1024–1034. https://doi.org/10.1038/nn.2172
Hampton DW, Innes N, Merkler D, Zhao C, Franklin RJ, Chandran S (2012) Focal immune-mediated white matter demyelination reveals an age-associated increase in axonal vulnerability and decreased remyelination efficiency. Am J Pathol 180(5):1897–1905. https://doi.org/10.1016/j.ajpath.2012.01.018
Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil MT, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lütjohann D, Möbius W, Simons M (2018) Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359(6376):684–688. https://doi.org/10.1126/science.aan4183
Sim FJ, Zhao C, Penderis J, Franklin RJ (2002) The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci 22(7):2451–2459. https://doi.org/10.1523/jneurosci.22-07-02451.2002
Neumann B, Baror R, Zhao C, Segel M, Dietmann S, Rawji KS, Foerster S, McClain CR, Chalut K, van Wijngaarden P, Franklin RJM (2019) Metformin restores cns remyelination capacity by rejuvenating aged stem cells. Cell Stem Cell 25(4):473-485.e478. https://doi.org/10.1016/j.stem.2019.08.015
Fennema D, Phillips IR, Shephard EA (2016) Trimethylamine and trimethylamine N-oxide, a flavin-containing monooxygenase 3 (FMO3)-mediated host-microbiome metabolic axis implicated in health and disease. Drug Metab Dispos 44(11):1839–1850. https://doi.org/10.1124/dmd.116.070615
Zhang AQ, Mitchell SC, Smith RL (1999) Dietary precursors of trimethylamine in man: a pilot study. Food Chem Toxicol 37(5):515–520. https://doi.org/10.1016/s0278-6915(99)00028-9
Zeisel SH, Mar MH, Howe JC, Holden JM (2003) Concentrations of choline-containing compounds and betaine in common foods. J Nutr 133(5):1302–1307. https://doi.org/10.1093/jn/133.5.1302
Smith JL, Wishnok JS, Deen WM (1994) Metabolism and excretion of methylamines in rats. Toxicol Appl Pharmacol 125(2):296–308. https://doi.org/10.1006/taap.1994.1076
Chen W, Wang S, Wu Y, Shen X, Guo Z, Li Q, Xing D (2020) Immunogenic cell death: A link between gut microbiota and anticancer effects. Microb Pathog 141:103983. https://doi.org/10.1016/j.micpath.2020.103983
Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, Ugarte E, Muñoz-Tamayo R, Paslier DL, Nalin R, Dore J, Leclerc M (2009) Towards the human intestinal microbiota phylogenetic core. Environ Microbiol 11(10):2574–2584. https://doi.org/10.1111/j.1462-2920.2009.01982.x
Subramaniam S, Fletcher C (2018) Trimethylamine N-oxide: breathe new life. Br J Pharmacol 175(8):1344–1353. https://doi.org/10.1111/bph.13959
Falony G, Vieira-Silva S, Raes J (2015) Microbiology meets big data: the case of gut microbiota-derived trimethylamine. Annu Rev Microbiol 69:305–321. https://doi.org/10.1146/annurev-micro-091014-104422
Unemoto T, Hayashi M, Miyaki K, Hayashi M (1966) Formation of trimethylamine from DL-carnitine by serratia marcescens. Biochem Biophys Acta 121(1):220–222. https://doi.org/10.1016/0304-4165(66)90382-5
Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, Tang WHW, DiDonato JA, Lusis AJ, Hazen SL (2014) γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab 20(5):799–812. https://doi.org/10.1016/j.cmet.2014.10.006
Athawale MV, Dordick JS, Garde S (2005) Osmolyte trimethylamine-N-oxide does not affect the strength of hydrophobic interactions: origin of osmolyte compatibility. Biophys J 89(2):858–866. https://doi.org/10.1529/biophysj.104.056671
Cho SS, Reddy G, Straub JE, Thirumalai D (2011) Entropic stabilization of proteins by TMAO. J Phys Chem B 115(45):13401–13407. https://doi.org/10.1021/jp207289b
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217(4566):1214–1222. https://doi.org/10.1126/science.7112124
Robinson DR, Jencks WP (1965) The effect of compounds of the urea-guanidinium class on the activity coefficient of acetyltetraglycine ethyl ester and related compounds. J Am Chem Soc 87:2462–2470. https://doi.org/10.1021/ja01089a028
O’Brien EP, Dima RI, Brooks B, Thirumalai D (2007) Interactions between hydrophobic and ionic solutes in aqueous guanidinium chloride and urea solutions: lessons for protein denaturation mechanism. J Am Chem Soc 129(23):7346–7353. https://doi.org/10.1021/ja069232+
Auton M, Holthauzen LM, Bolen DW (2007) Anatomy of energetic changes accompanying urea-induced protein denaturation. Proc Natl Acad Sci USA 104(39):15317–15322. https://doi.org/10.1073/pnas.0706251104
Hand SC, Somero GN (1982) Urea and methylamine effects on rabbit muscle phosphofructokinase catalytic stability and aggregation state as a function of pH and temperature. J Biol Chem 257(2):734–741
Hand SC, Carpenter JF (1986) pH-Induced hysteretic properties of phosphofructokinase purified from rat myocardium. Am J Physiol 250(3 Pt 2):R505-511. https://doi.org/10.1152/ajpregu.1986.250.3.R505
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472(7341):57–63. https://doi.org/10.1038/nature09922
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL (2013) Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19(5):576–585. https://doi.org/10.1038/nm.3145
Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM (2015) The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep 10(3):326–338. https://doi.org/10.1016/j.celrep.2014.12.036
Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ (2013) Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 17(1):49–60. https://doi.org/10.1016/j.cmet.2012.12.011
Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL (2016) Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165(1):111–124. https://doi.org/10.1016/j.cell.2016.02.011
Din AU, Hassan A, Zhu Y, Yin T, Gregersen H, Wang G (2019) Amelioration of TMAO through probiotics and its potential role in atherosclerosis. Appl Microbiol Biotechnol 103(23–24):9217–9228. https://doi.org/10.1007/s00253-019-10142-4
Collins HL, Drazul-Schrader D, Sulpizio AC, Koster PD, Williamson Y, Adelman SJ, Owen K, Sanli T, Bellamine A (2016) L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis 244:29–37. https://doi.org/10.1016/j.atherosclerosis.2015.10.108
Shi W, Huang Y, Yang Z, Zhu L, Yu B (2021) Reduction of TMAO level enhances the stability of carotid atherosclerotic plaque through promoting macrophage M2 polarization and efferocytosis. Biosci Rep 41:6. https://doi.org/10.1042/bsr20204250
Huc T, Drapala A, Gawrys M, Konop M, Bielinska K, Zaorska E, Samborowska E, Wyczalkowska-Tomasik A, Pączek L, Dadlez M, Ufnal M (2018) Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am J Physiol Heart Circ Physiol 315(6):H1805-h1820. https://doi.org/10.1152/ajpheart.00536.2018
Ge X, Zheng L, Zhuang R, Yu P, Xu Z, Liu G, Xi X, Zhou X, Fan H (2020) The gut microbial metabolite trimethylamine N-oxide and hypertension risk: a systematic review and dose-response meta-analysis. Adv Nutr 11(1):66–76. https://doi.org/10.1093/advances/nmz064
Jiang S, Shui Y, Cui Y, Tang C, Wang X, Qiu X, Hu W, Fei L, Li Y, Zhang S, Zhao L, Xu N, Dong F, Ren X, Liu R, Persson PB, Patzak A, Lai EY, Wei Q, Zheng Z (2021) Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol 46:102115. https://doi.org/10.1016/j.redox.2021.102115
Zhao ZH, Xin FZ, Zhou D, Xue YQ, Liu XL, Yang RX, Pan Q, Fan JG (2019) Trimethylamine N-oxide attenuates high-fat high-cholesterol diet-induced steatohepatitis by reducing hepatic cholesterol overload in rats. World J Gastroenterol 25(20):2450–2462. https://doi.org/10.3748/wjg.v25.i20.2450
Chen YM, Liu Y, Zhou RF, Chen XL, Wang C, Tan XY, Wang LJ, Zheng RD, Zhang HW, Ling WH, Zhu HL (2016) Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep 6:19076. https://doi.org/10.1038/srep19076
Chen PY, Li S, Koh YC, Wu JC, Yang MJ, Ho CT, Pan MH (2019) Oolong tea extract and citrus peel polymethoxyflavones reduce transformation of l-carnitine to trimethylamine-N-oxide and decrease vascular inflammation in l-carnitine feeding mice. J Agric Food Chem 67(28):7869–7879. https://doi.org/10.1021/acs.jafc.9b03092
Dumas ME, Rothwell AR, Hoyles L, Aranias T, Chilloux J, Calderari S, Noll EM, Péan N, Boulangé CL, Blancher C, Barton RH, Gu Q, Fearnside JF, Deshayes C, Hue C, Scott J, Nicholson JK, Gauguier D (2017) Microbial-host co-metabolites are prodromal markers predicting phenotypic heterogeneity in behavior, obesity, and impaired glucose tolerance. Cell Rep 20(1):136–148. https://doi.org/10.1016/j.celrep.2017.06.039
Nowiński A, Ufnal M (2018) Trimethylamine N-oxide: a harmful, protective or diagnostic marker in lifestyle diseases? Nutrition 46:7–12. https://doi.org/10.1016/j.nut.2017.08.001
Hoyles L, Pontifex MG, Rodriguez-Ramiro I, Anis-Alavi MA, Jelane KS, Snelling T, Solito E, Fonseca S, Carvalho AL, Carding SR, Müller M, Glen RC, Vauzour D, McArthur S (2021) Regulation of blood-brain barrier integrity by microbiome-associated methylamines and cognition by trimethylamine N-oxide. Microbiome 9(1):235. https://doi.org/10.1186/s40168-021-01181-z
Jia J, Dou P, Gao M, Kong X, Li C, Liu Z, Huang T (2019) Assessment of causal direction between gut microbiota-dependent metabolites and cardiometabolic health: a bidirectional Mendelian randomization analysis. Diabetes 68(9):1747–1755. https://doi.org/10.2337/db19-0153
Winther SA, Øllgaard JC, Tofte N, Tarnow L, Wang Z, Ahluwalia TS, Jorsal A, Theilade S, Parving HH, Hansen TW, Hazen SL, Pedersen O, Rossing P (2019) Utility of plasma concentration of trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes Care 42(8):1512–1520. https://doi.org/10.2337/dc19-0048
Zhou J, Wang D, Li B, Li X, Lai X, Lei S, Li N, Zhang X (2021) Relationship between plasma trimethylamine N-oxide levels and renal dysfunction in patients with hypertension. Kidney Blood Press Res 46(4):421–432. https://doi.org/10.1159/000513033
Gessner A, di Giuseppe R, Koch M, Fromm MF, Lieb W, Maas R (2020) Trimethylamine-N-oxide (TMAO) determined by LC-MS/MS: distribution and correlates in the population-based popgen cohort. Clin Chem Lab Med 58(5):733–740. https://doi.org/10.1515/cclm-2019-1146
Romano KA, Vivas EI, Amador-Noguez D, Rey FE (2015) Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio 6:2. https://doi.org/10.1128/mBio.02481-14
Hartiala J, Bennett BJ, Tang WH, Wang Z, Stewart AF, Roberts R, McPherson R, Lusis AJ, Hazen SL, Allayee H (2014) Comparative genome-wide association studies in mice and humans for trimethylamine N-oxide, a proatherogenic metabolite of choline and l-carnitine. Arterioscler Thromb Vasc Biol 34(6):1307–1313. https://doi.org/10.1161/atvbaha.114.303252
Li T, Chen Y, Gua C, Li X (2017) Elevated circulating trimethylamine n-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front Physiol 8:350. https://doi.org/10.3389/fphys.2017.00350
Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL (2014) Measurement of trimethylamine-N-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem 455:35–40. https://doi.org/10.1016/j.ab.2014.03.016
Vogelzang NJ, Porta C, Mutti L (2005) New agents in the management of advanced mesothelioma. Semin Oncol 32(3):336–350. https://doi.org/10.1053/j.seminoncol.2005.02.010
Johri A, Chandra A, Flint Beal M (2013) PGC-1α, mitochondrial dysfunction, and Huntington’s disease. Free Radical Biol Med 62:37–46. https://doi.org/10.1016/j.freeradbiomed.2013.04.016
Li D, Ke Y, Zhan R, Liu C, Zhao M, Zeng A, Shi X, Ji L, Cheng S, Pan B, Zheng L, Hong H (2018) Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 17(4):e12768. https://doi.org/10.1111/acel.12768
Brunt VE, LaRocca TJ, Bazzoni AE, Sapinsley ZJ, Miyamoto-Ditmon J, Gioscia-Ryan RA, Neilson AP, Link CD, Seals DR (2021) The gut microbiome-derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience 43(1):377–394. https://doi.org/10.1007/s11357-020-00257-2
Mueed Z, Mehta D, Rai PK, Kamal MA, Poddar NK (2020) Cross-interplay between osmolytes and mTOR in Alzheimer’s disease pathogenesis. Curr Pharm Des 26(37):4699–4711. https://doi.org/10.2174/1381612826666200518112355
Xu R, Wang Q (2016) Towards understanding brain-gut-microbiome connections in Alzheimer’s disease. BMC Syst Biol 10:63. https://doi.org/10.1186/s12918-016-0307-y
Arrona Cardoza P, Spillane MB, Morales Marroquin E (2022) Alzheimer’s disease and gut microbiota: does trimethylamine N-oxide (TMAO) play a role? Nutr Rev 80(2):271–281. https://doi.org/10.1093/nutrit/nuab022
Kumari A, Rajput R, Shrivastava N, Somvanshi P, Grover A (2018) Synergistic approaches unraveling regulation and aggregation of intrinsically disordered β-amyloids implicated in Alzheimer’s disease. Int J Biochem Cell Biol 99:19–27. https://doi.org/10.1016/j.biocel.2018.03.014
Yang DS, Yip CM, Huang TH, Chakrabartty A, Fraser PE (1999) Manipulating the amyloid-beta aggregation pathway with chemical chaperones. J Biol Chem 274(46):32970–32974. https://doi.org/10.1074/jbc.274.46.32970
Canchi DR, Jayasimha P, Rau DC, Makhatadze GI, Garcia AE (2012) Molecular mechanism for the preferential exclusion of TMAO from protein surfaces. J Phys Chem B 116(40):12095–12104. https://doi.org/10.1021/jp304298c
Ma J, Pazos IM, Gai F (2014) Microscopic insights into the protein-stabilizing effect of trimethylamine N-oxide (TMAO). Proc Natl Acad Sci USA 111(23):8476–8481. https://doi.org/10.1073/pnas.1403224111
Buawangpong N, Pinyopornpanish K, Siri-Angkul N, Chattipakorn N, Chattipakorn SC (2021) The role of trimethylamine-N-Oxide in the development of Alzheimer’s disease. J Cell Physiol. https://doi.org/10.1002/jcp.30646
Gao Q, Wang Y, Wang X, Fu S, Zhang X, Wang RT, Zhang X (2019) Decreased levels of circulating trimethylamine N-oxide alleviate cognitive and pathological deterioration in transgenic mice: a potential therapeutic approach for Alzheimer’s disease. Aging 11(19):8642–8663. https://doi.org/10.18632/aging.102352
Govindarajulu M, Pinky PD, Steinke I, Bloemer J, Ramesh S, Kariharan T, Rella RT, Bhattacharya S, Dhanasekaran M, Suppiramaniam V, Amin RH (2020) Gut metabolite tmao induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Front Mol Neurosci 13:138. https://doi.org/10.3389/fnmol.2020.00138
Lanz M, Janeiro MH, Milagro FI, Puerta E, Ludwig IA, Pineda-Lucena A, Ramírez MJ, Solas M (2022) Trimethylamine N-oxide (TMAO) drives insulin resistance and cognitive deficiencies in a senescence accelerated mouse model. Mech Ageing Dev 204:111668. https://doi.org/10.1016/j.mad.2022.111668
Guo JD, Zhao X, Li Y, Li GR, Liu XL (2018) Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease (Review). Int J Mol Med 41(4):1817–1825. https://doi.org/10.3892/ijmm.2018.3406
Chou RH, Chen CY, Chen IC, Huang HL, Lu YW, Kuo CS, Chang CC, Huang PH, Chen JW, Lin SJ (2019) Trimethylamine N-oxide, circulating endothelial progenitor cells, and endothelial function in patients with stable angina. Sci Rep 9(1):4249. https://doi.org/10.1038/s41598-019-40638-y
Chung SJ, Rim JH, Ji D, Lee S, Yoo HS, Jung JH, Baik K, Choi Y, Ye BS, Sohn YH, Yun M, Lee SG, Lee PH (2021) Gut microbiota-derived metabolite trimethylamine N-oxide as a biomarker in early Parkinson’s disease. Nutrition 83:111090. https://doi.org/10.1016/j.nut.2020.111090
Jamal S, Kumari A, Singh A, Goyal S, Grover A (2017) Conformational ensembles of alpha-synuclein derived peptide with different osmolytes from temperature replica exchange sampling. Front Neurosci 11:684. https://doi.org/10.3389/fnins.2017.00684
Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368(17):1575–1584. https://doi.org/10.1056/NEJMoa1109400
Brugère JF, Borrel G, Gaci N, Tottey W, O’Toole PW, Malpuech-Brugère C (2014) Archaebiotics: proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes 5(1):5–10. https://doi.org/10.4161/gmic.26749
Velasquez MT, Ramezani A, Manal A, Raj DS (2016) Trimethylamine N-oxide: the good, the bad and the unknown. Toxins 8:11. https://doi.org/10.3390/toxins8110326
Barrett EL, Kwan HS (1985) Bacterial reduction of trimethylamine oxide. Annu Rev Microbiol 39:131–149. https://doi.org/10.1146/annurev.mi.39.100185.001023
Shih DM, Wang Z, Lee R, Meng Y, Che N, Charugundla S, Qi H, Wu J, Pan C, Brown JM, Vallim T, Bennett BJ, Graham M, Hazen SL, Lusis AJ (2015) Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res 56(1):22–37. https://doi.org/10.1194/jlr.M051680
Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL (2015) Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163(7):1585–1595. https://doi.org/10.1016/j.cell.2015.11.055
Kuka J, Liepinsh E, Makrecka-Kuka M, Liepins J, Cirule H, Gustina D, Loza E, Zharkova-Malkova O, Grinberga S, Pugovics O, Dambrova M (2014) Suppression of intestinal microbiota-dependent production of pro-atherogenic trimethylamine N-oxide by shifting l-carnitine microbial degradation. Life Sci 117(2):84–92. https://doi.org/10.1016/j.lfs.2014.09.028
Zhu Y, Li Q, Jiang H (2020) Gut microbiota in atherosclerosis: focus on trimethylamine N-oxide. APMIS 128(5):353–366. https://doi.org/10.1111/apm.13038
Verhaar BJH, Prodan A, Nieuwdorp M, Muller M (2020) Gut microbiota in hypertension and atherosclerosis: a review. Nutrients 12:10. https://doi.org/10.3390/nu12102982
Zeisel SH, Warrier M (2017) Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr 37:157–181. https://doi.org/10.1146/annurev-nutr-071816-064732
Chen ML, Long Yi, Zhang Y, Zhou X, Ran L, Yang J, Zhu JD, Zhang QY, Mi MT (2016) Resveratrol attenuates trimethylamine-N-oxide tmao-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio 7(2):02210–02215. https://doi.org/10.1128/mBio.02210-15
Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature 488(7410):178–184. https://doi.org/10.1038/nature11319
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13(10):701–712. https://doi.org/10.1038/nrn3346
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155(7):1451–1463. https://doi.org/10.1016/j.cell.2013.11.024
Funding
The present study was supported by the National Natural Science Foundation of China (Grants 81671166, 81571151, 81601140, and 81641039), and Fundamental Research Funds for the Central Universities of Central South University (grant 2021zzts1033, 2021zzts1029).
Author information
Authors and Affiliations
Contributions
They were involved in writing the paper and had final approval of the submitted and published versions.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, L., Yu, F. & Xia, J. Trimethylamine N-oxide: role in cell senescence and age-related diseases. Eur J Nutr 62, 525–541 (2023). https://doi.org/10.1007/s00394-022-03011-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03011-w