Abstract
Purpose
The main aim of the present study was to examine the effect of a fish protein supplement made from by-products from production of Atlantic salmon, on blood concentration of micronutrients.
Methods
We conducted an 8-week double-blind parallel-group randomised controlled trial. In total, 88 adults were randomised to a salmon fish protein supplement or placebo, and 74 participants were included in the analysis of vitamin D, omega-3, vitamin B12, selenium, folate, zinc, homocysteine and mercury.
Results
During the intervention period, geometric mean (GSD) of serum vitamin B12 concentrations increased from 304 (1.40) to 359 (1.42) pmol/L in the fish protein group (P vs. controls = 0.004) and mean (SD) serum selenium increased from 1.18 (0.22) to 1.30 (0.20) μmol/L (P vs. controls = 0.002). The prevalence of low vitamin B12 status (B12 < 148–221 > pmol/L) decreased from 15.4 to 2.6% in the fish protein group, while increasing from 5.9 to 17.6% in the placebo group (P = 0.045). There was no difference between the groups in serum levels of the other micronutrients measured.
Conclusion
Including a salmon fish protein supplement in the daily diet for 8 weeks, increases serum vitamin B12 and selenium concentrations. From a sustainability perspective, by-products with high contents of micronutrients and low contents of contaminants, could be a valuable dietary supplement or food ingredient in populations with suboptimal intake.
Trail Registration
The study was registered at ClinicalTrials.gov (ID: NCT03764423) on June 29th 2018.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most dietary guidelines recommend including fish in the diet [1]. The beneficial effects of fish intake have largely been attributed to omega-3 polyunsaturated fatty acids present in fatty fish [2]. However, fish contain other nutrients such as taurine, vitamin D, vitamin B12 (B12), iodine and selenium [3], and intervention studies show that regular consumption of fish increases concentrations of serum vitamin D (s-vitamin D) and B12 (s-B12), urinary iodine and plasma selenium (p-selenium) [4,5,6]. Worldwide, there are concerns regarding intake and status of several of the micronutrients related to fish intake [7,8,9,10].
Norway is one of the world’s largest aquaculture and fishing nations, and the Norwegian fish industry generates a large amount of materials discarded as by-products, mainly utilized for animal feed production [11]. In 2018, only 13% of the by-products were used for human consumption [11]. From a sustainability perspective, it is important to explore available food resources at our disposal. With the expected growth in the aquaculture industry, by-products will become even more available. By-products from fish contain protein, lipids and minerals such as calcium, phosphate, zinc, selenium and iron [12]. Such by-products should ideally be utilized for human consumption [13], e.g. as a novel food ingredient [14].
We have previously reported the effects of eight weeks of daily intake of a fish protein supplement made from by-products from production of Atlantic salmon on cardiometabolic risk markers in adults with increased risk of type 2 diabetes mellitus. We found no beneficial effect on markers related to glucose tolerance, serum lipids, weight or blood pressure compared to the placebo group [15]. In the present secondary analysis, the main aim was to examine the effect on blood concentration of micronutrients related to fish intake or abundant in fish by-products. Additionally, we compared the content of micronutrients and contaminants in the salmon fish protein supplement to the recommended intake (RI) of micronutrients in the Nordic Nutrition Recommendations (NNR) [16] and the tolerable weekly intake (TWI) levels of contaminants set by the European Food Safety Authorities Panel on Contaminants in the Food Chain (EFSA CONTAM Panel) [17,18,19,20,21].
Methods
Ethics
The study was conducted according to the guidelines laid down in the Declaration of Helsinki. All participants gave their written informed consent, and the Regional Ethics Committee for Medical Research in South East Norway approved the study (2018/749/REK sør-øst A). The study was registered at ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT03764423).
Participants
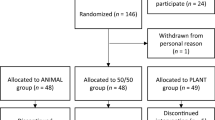
The present study is based on a previously performed randomised controlled trial, the FishMeal human intervention study, where the primary outcome was changes from baseline in serum glucose (s-glucose) measured after a 2-h oral glucose tolerance test (OGTT). The description of participants, study setting and protocol for study visits have been published in detail previously [15]. In brief, the study was conducted at the University of Oslo, Norway from August 2018 until September 2019. We included weight-stable adults with elevated blood glucose (fasting s-glucose ≥ 5.6 mmol/l, 2 h-OGTT-s-glucose ≥ 6.5 mmol/l or HbA1c ≥ 40 mmol/mol). Exclusion criteria were type 1 and 2 diabetes mellitus, age-related elevated blood pressure, use of glucose lowering drugs, drugs related to inflammation and systemic use of corticosteroids, or unstable use of lipid lowering drugs, thyroxine, blood pressure lowering drugs and drugs affecting appetite. Further exclusion criteria were fish/seafood intake exceeding 450 g/week and allergy to fish or shellfish. Daily users of protein supplement powder, and participants who were pregnant, breastfeeding or planning pregnancy were also excluded. Participants with sporadic/unstable use of dietary supplements were excluded, but participants with regular/stable use of dietary supplement were included.
Study design
We conducted an 8-week double-blind, randomised controlled parallel trial comparing a salmon fish protein supplement with placebo on blood concentration of micronutrients. Before study start, all participants performed a 2–4-week run-in period with a maximum intake of 150 g of fish and seafood per week. Participants were stratified by sex and age (< 50 y, ≥ 50 y) prior to a block randomisation done by an external statistician. The experimental group received capsules containing salmon fish protein, microcrystalline cellulose, antioxidants and excipients. The placebo group received capsules containing microcrystalline cellulose and antioxidants and excipients similar to the fish protein capsules. We instructed participants in both groups to consume ten capsules together with a meal three times per day for 8 weeks. Capsules were packed in blister sheets with one daily dose per sheet, and delivered in boxes with one weekly dose per box. Participants were instructed to deliver all blister sheets, both empty and full, at the end-of-study visit. Additionally, the participants received a registration scheme to register their intake of capsules. Compliance was assessed by counting the number of capsules consumed during the intervention period, divided by the number of capsules scheduled for the intervention period. All participants had a compliance > 70% [15]. We advised the participants to maintain their usual lifestyle habits throughout the study, including physical activity, supplement use and diet, except for a reduction in fish and seafood intake to a maximum of 150 g per week. Habitual dietary intake was assessed prior to the intervention through a semi-quantitative food frequency questionnaire (FFQ) designed to capture dietary habits during the last year [22]. The same FFQ was used to assess the participants’ diets during the 8-week intervention.
Study product
The fish protein supplement was made from by-products from production of Atlantic salmon. Content of nutrients and contaminants was analysed before encapsulation (Eurofins Food & Feed Testing Norway AS, Moss, Norway). To evaluate the content of micronutrients, we compared the contribution from a daily dose of capsules to recommended intake (RI) in the Nordic Nutrition Recommendations (NNR) for vitamin D, B12, iodine, selenium, riboflavin, folate, calcium, phosphorous, magnesium, iron and zinc [16]. We used male adults as a reference population. To evaluate the content of contaminants, we compared the contribution from a weekly dose of capsules to the tolerable weekly intake (TWI) levels per kg body weight set by the EFSA CONTAM Panel for arsenic, lead, cadmium, mercury and dioxins and dioxin-like polychlorinated biphenyls (dl-PCBs) [17,18,19,20,21]. We used 70 kg as a reference weight, as recommended by the EFSA Scientific Committee [23]. As no TWI exist for copper, we compared the contribution from a weekly dose of capsules to the tolerable upper intake level (UL) [16].
Blood sampling and standard laboratory analysis
Venous blood samples were drawn after an overnight fast (≥ 10 h). Participants were instructed to avoid drinking alcohol and doing strenuous physical activity the day before blood sampling. Serum was obtained from silica gel tubes and Lithium Heparin gel tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and kept at room temperature for > 30–60 < min, until centrifugation (1500×g, 15 min). Plasma was obtained from K2EDTA tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), immediately placed on ice, and centrifuged within 10 min (2000×g, 4 °C, 15 min). Lithium Heparin tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) with whole blood were kept at room temperature. Concentrations of s-B12, s-folate and p-homocysteine were measured on an ADVIA Centaur XPT Immunoassay System analyser (Siemens Healthineers, Tarrytown, NY, USA); s-vitamin D was measured on a Water Acquity UPLC analyser (Waters, Milford, MA, USA); and s-selenium, s-zinc and whole blood mercury were measured on a Nexion 2000 ICP-MS (Perkin Elmer, Shelton, CT, USA) by standard methods at an accredited routine laboratory (Fürst Medical Laboratory, Oslo, Norway). P-omega-3 was measured using a high-throughput nuclear magnetic resonance spectroscopy platform (Nightingale Health, Helsinki, Finland).
Statistical analysis
Power calculation was originally conducted with the aim to investigate the effect on s-glucose 2 h after an OGTT. We estimated that 120 participants (including a 20% dropout rate) were required to obtain 80% power with a type I error of 5% to detect a clinically relevant difference between the two groups of 0.4 mmol/l (standard deviation (SD) 0.7).
Data are presented as mean (SD) or median (quartiles, Q1–Q3) for continuous variables or as frequency (percentage, %) for categorical variables. Differences between the groups were tested with a linear regression model (outcome variable ~ intervention group + outcome variable at baseline), hereafter called crude model. We performed the same analysis adjusting for strata (age and sex) in addition to the outcome variable at baseline (outcome variable ~ intervention group + outcome variable at baseline + age + sex), hereafter called the adjusted model. Skewed variables (s-B12, s-folate, p-omega-3 and whole blood mercury) were log-transformed before analysis. Results from the regression analysis are presented as β-coefficient with 95% confidence interval (CI) or logβ-coefficient with 95% CI for skewed variables (s-B12, s-folate, p-omega-3 and whole blood mercury). Baseline and end-of-study values for skewed variables are presented as geometric mean and geometric standard deviation (GSD). P < 0.05 was considered significant. The models were checked for independence and normality of the residuals. Differences in B12 status (low B12 status (B12 < 148–221 > pmol/L) and B12 deficiency (B12 < 148 pmol/L)) were tested using two-sided Fisher’s exact test. One participant was excluded from the analysis of s-B12, p-homocysteine and B12 status due to B12-injection during the intervention period.
Statistical analysis were performed in Stata/MP 16.0 (StataCorp LLC, College Station, TX, USA) [24] and the figure was created in R (R Foundation for Statistical Computing, Vienna, Austria) [25].
Results
In total, 717 participants were assessed for eligibility, 88 were randomly assigned, 83 received allocated interventions and 7 were lost to follow-up. Thus, 76 participants completed the study. Two participants were non-compliant with the study protocol, leaving a total of 74 participants included in the statistical analysis, as previously reported [15]. The participants were 56 (Q1–Q3: 48–64) years of age, with a BMI of 33.1 (Q1–Q3: 29.9–36.1) kg/m2 and 64% were female. Further participant characteristics and information on dietary intake is summarized in Table 1.
Content of micronutrients and contaminants in the fish protein supplement
Content of nutrients and contaminants in the fish protein supplement is summarized in Tables 2 and 3. One daily dose of the fish protein supplement provided 4.5 μg B12, corresponding to 227% of the RI (Table 2). For other micronutrients measured in the study product, the content corresponded to 117% of the RI for zinc, 29% for selenium, 26% for phosphorous, 23% for calcium, 22% for riboflavin, 15% for folate, 9% for iron, 9% for iodine, 4% for vitamin D and 3% for magnesium.
Weekly intake of capsules did not exceed UL or TWI for any of the examined contaminants in the supplement for a person with a body weight of 70 kg (Table 3).
Effect of fish protein supplementation on blood concentrations of nutrients
Differences between the fish protein group and the placebo group on blood concentrations of nutrients using crude and adjusted models are shown in Table 4. During the 8-week intervention period, geometric mean (GSD) of s-B12 concentrations increased from 304 (1.40) to 359 (1.42) pmol/L in the fish protein group (P vs. controls = 0.004) in the adjusted analysis. Individual responses on s-B12 concentration is illustrated in Fig. 1.
Since we found a significant increase in s-B12 in the fish protein group vs. the placebo group, we investigated whether the supplement could affect B12 status based on established clinical cut-offs. The results are summarized in Table 5. At baseline, there were no difference between the groups in prevalence of low B12 status (B12 < 148–221 > pmol/L) (P = 0.271). At the end-of-study, 2.6% of the participants in the fish protein group and 17.6% in the placebo group had low B12 status (P vs. controls = 0.045). None of the participants had B12 deficiency (B12 < 148 pmol/L) at either time point.
During the intervention period, mean (SD) s-selenium increased from 1.18 (0.22) to 1.30 (0.20) μmol/L in the fish protein group (P vs. controls = 0.002). S-folate, s-vitamin D, p-omega-3, s-zinc, p-homocysteine or whole blood mercury did not change during the intervention period.
Discussion
In the present study, we examined the effect of including a fish protein supplement made from by-products from production of Atlantic salmon in the daily diet for 8 weeks, on blood concentration of micronutrients. We found that 8 weeks of fish protein supplementation, which was a good source of micronutrients and had a low content of contaminants, increased serum concentrations of B12 and selenium among adults. To the best of our knowledge, this is the first clinical trial examining the effects of a fatty fish protein supplement on blood concentration of micronutrients.
Although the study product in the present study was made from fatty fish, we did not find an effect on vitamin D and omega-3, despite omega-3 being a well-established biomarker of fatty fish intake [26]. P-omega-3 increases in a dose-dependent manner, however, most studies examining this relationship have provided higher doses of EPA and/or DHA than the study product, providing 151 mg/d of EPA and DHA [27]. Additionally, about one third of the participants in the present study used omega-3 supplements regularly prior to the study and during the intervention (data not shown). As the daily dose of the study product provided only 0.4 μg vitamin D, we did not expect an increase in s-vitamin D. Additionally, since many were using supplements with vitamin D regularly (data not shown), we did not expect a decrease in s-vitamin D. In an 8-week RCT in adults (n = 63) with overweight or obesity comparing intake of salmon or cod consumed as whole filet, 750 g/week of salmon was not sufficient to prevent a decrease in s-vitamin D in autumn in South-Western Norway, despite a median vitamin D intake of 11.9 μg/d [28], which was above the recommendation of 10 µg/d [16].
Our findings on s-B12 and s-selenium are in line with intervention studies on intake of fish consumed as whole filet. A randomised cross-over study in overweight men (n = 32) comparing herring with chicken and pork (750 g/week for 6 weeks) found an increase in s-B12 (8.9%) and s-selenium (4.6%), but not s-vitamin D, during the herring diet [5]. In the previously mentioned RCT comparing intake of salmon or cod consumed as whole filet (750 g/week), only cod increased s-selenium compared to controls [29]. We have previously performed a post-prandial analysis of serum amino acids in five healthy adults after intake of the study product, and found a non-significant increase in serum levels of most amino acids [15], supporting that the capsules were absorbed. Both B12 and selenium have high bioavailability and serum and plasma concentrations reflects dietary exposure [7, 30], supporting our findings in the present study.
In line with our findings on increased s-B12 concentration, participants in the salmon fish protein group, but not in the placebo group, improved their B12 status during the intervention period. Although we did not detect any participants with B12 deficiency in the present study, 11% had low B12 status at baseline in the two groups combined. However, although s-B12 is a useful indicator of B12 status, it lacks sensitivity and specificity for diagnosing B12 deficiency [7].
Although the worldwide prevalence of B12 deficiency is uncertain, it is estimated to exceed 40% in subpopulations such as children, women of childbearing age and older adults in low- and middle-income countries [7]. In Europe, existing prevalence data on B12 deficiency mostly focus on the elderly, and varies from 5.9% in a Norwegian study [31] to 24% in a study from the Netherlands [32]. Less is known about selenium status. However, low selenium intake is reported in several European countries, as selenium content in food is dependent on contents in the soil the food is produced and the selenium contents of European soil is generally low [33]. It is estimated that 40% of pregnant women in the Norwegian mother and child cohort had an inadequate selenium intake [34].
In a study on preschool children (n = 210), consumption of fatty fish three times per week for 16 weeks resulted in higher concentrations of hair mercury compared to meat [35], even though the exposure for fish mercury from the intervention did not exceed the TWI. Similarly, the contribution from a weekly dose of capsules in the present study did not exceed the TWI. Benefit-risk assessments of seafood conclude that the benefits outweigh the potential harmful effects from contaminants [36, 37]. Both selenium and zinc were abundant in the study product in the present study, and have the potential to antagonize toxic effects of heavy metals [38].
The strengths of the present fish protein human intervention study are the randomised controlled double-blind design, while the main limitation is that power calculation was done for the primary endpoint in the original study, and not the outcomes in the present study. To avoid overestimation of the contribution of micronutrients to the RI from a daily dose of capsules, we used male adult as a reference population, as RI for men are the same or higher as those for women, except from iron. Additionally, RI for several micronutrients is higher during pregnancy and breastfeeding [16]. Thus, another limitation is that different assessments must be done to evaluate the potential use of the study product in other populations.
Low food intake in general or low intake of foods of animal origin, may cause low intake of several nutrients abundant in the study product, such as vitamin B12, zinc and selenium. To ensure sufficient intake of these nutrients in such populations, one possibility is to use by-products from fish as a dietary supplement or food ingredient. A study examining the sensory acceptance of bread baked with added fish flour, found that the acceptance was better than, or as good as, the bread without fish flour. Additionally, the bread with fish flour had higher contents of protein, essential fatty acids, and minerals, but lower content of carbohydrates [39].
In conclusion, in the present study, a fish protein supplement made from by-products from production of Atlantic salmon included in the daily diet for 8 weeks, increased serum vitamin B12 and selenium concentrations. From a sustainability perspective, by-products with high contents of micronutrients and low contents of contaminants, as in the present study, could be a valuable dietary supplement or food ingredient in populations with suboptimal intake. The potential use of by-products from the aquaculture and fishery industry for human consumption should, therefore, be further elucidated.
Data availability
Anonymised data used in the manuscript will be available upon request.
Code availability
Stata/MP 16.0 (StataCorp LLC, College Station, TX, USA).
References
Cámara M, Giner RM, González-Fandos E, López-García E, Mañes J, Portillo MP, Rafecas M, Domínguez L, Martínez JA (2021) Food-based dietary guidelines around the world: a comparative analysis to update AESAN scientific committee dietary recommendations. Nutrients. https://doi.org/10.3390/nu13093131
Mozaffarian D, Wu JHY (2011) Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. JACC 58:2047–2067. https://doi.org/10.1016/j.jacc.2011.06.0633.
Lund EK (2013) Health benefits of seafood; is it just the fatty acids? Food Chem 140:413–420. https://doi.org/10.1016/j.foodchem.2013.01.034
Lehmann U, Gjessing HR, Hirche F, Mueller-Belecke A, Gudbrandsen OA, Ueland PM, Mellgren G, Lauritzen L, Lindqvist H, Hansen AL, Erkkilä AT, Pot GK, Stangl GI, Dierkes J (2015) Efficacy of fish intake on vitamin D status: a meta-analysis of randomized controlled trials. Am J Clin Nutr 102:837–847. https://doi.org/10.3945/ajcn.114.105395
Scheers N, Lindqvist H, Langkilde AM, Undeland I, Sandberg AS (2014) Vitamin B12 as a potential compliance marker for fish intake. Eur J Nutr 53:1327–1333. https://doi.org/10.1007/s00394-013-0632-5
Øyen J, Aadland EK, Liaset B, Fjære E, Dahl L, Madsen L (2021) Lean-seafood intake increases urinary iodine concentrations and plasma selenium levels: a randomized controlled trial with crossover design. Eur J Nutr 60:1679–1689. https://doi.org/10.1007/s00394-020-02366-2
Allen LH, Miller JW, de Groot L, Rosenberg IH, Smith AD, Refsum H, Raiten DJ (2018) Biomarkers of nutrition for development (BOND): vitamin B-12 review. J Nutr 148:1995–2027. https://doi.org/10.1093/jn/nxy201
Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Tmava-Berisha A, Martucci G, Pilz S, Malle O (2020) Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr 74:1498–1513. https://doi.org/10.1038/s41430-020-0558-y
Jones GD, Droz B, Greve P, Gottschalk P, McGrath SP, Seneviratne SI, Smith P, Winkel LHE (2017) Selenium deficiency risk predicted to increase under future climate change. PNAS 114:2848–2853. https://doi.org/10.1073/pnas.1611576114
Rohner F, Zimmermann M, Jooste P, Pandav C, Caldwell K, Raghavan R, Raiten DJ (2014) Biomarkers of nutrition for development–iodine review. J Nutr 144:1322–1342. https://doi.org/10.3945/jn.113.181974
Richardsen R, Myhre M, Nystøyl R, Strandheim G (2019) Analysis of marine by-products 2018. SINTEF Ocean AS and Kontali Analyse AS.
Al Khawli F, Martí-Quijal FJ, Ferrer E, Ruiz MJ, Berrada H, Gavahian M, Barba FJ, de la Fuente B (2020) Aquaculture and its by-products as a source of nutrients and bioactive compounds. Adv Food Nutr Res 92:1–33. https://doi.org/10.1016/bs.afnr.2020.01.001
Le Gouic AV, Harnedy PA, FitzGerald RJ (2018) Bioactive peptides from fish protein by-products. In: Mérillon JM, Ramawat K (eds) Bioactive molecules in food. Reference series in phytochemistry. Springer, Cham, pp 1–35
Shahidi F, Varatharajan V, Peng H, Senadheera R (2019) Utilization of marine by-products for the recovery of value-added products. J Food Bioact 6:10–61
Hustad KS, Ottestad I, Hjorth M, Dalen KT, Sæther T, Sheikh NA, Strømnes M, Ulven SM, Holven KB (2021) No effect of salmon fish protein on 2-h glucose in adults with increased risk of type 2 diabetes: a randomised controlled trial. Br J Nutr 126:1304–1313
Secretary of the Nordic Council of Ministers (2014) Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity. Copenhagen, Denmark: Nordic Council of Ministers.
EFSA Panel on Contaminants in the Food Chain (2011) Scientific opinion on tolerable weekly intake for cadmium. EFSA J 9:1975. https://doi.org/10.2903/j.efsa.2011.1975
EFSA Panel on Contaminants in the Food Chain (2009) Scientific opinion on arsenic in food. EFSA J 7:1351. https://doi.org/10.2903/j.efsa.2009.1351
EFSA Panel on Contaminants in the Food Chain (2010) Scientific opinion on lead in food. EFSA J 8:1570. https://doi.org/10.2903/j.efsa.2010.1570
EFSA Panel on Contaminants in the Food Chain (2012) Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J 10:2985. https://doi.org/10.2903/j.efsa.2012.2985
EFSA Panel on Contaminants in the Food Chain (2018) Scientific opinion on the risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J 16:5333. https://doi.org/10.2903/j.efsa.2018.2333
Carlsen MH, Karlsen A, Lillegaard ITL, Gran JM, Drevon CA, Blomhoff R, Andersen LF (2011) Relative validity of fruit and vegetable intake estimated from an FFQ, using carotenoid and flavonoid biomarkers and the method of triads. Br J Nutr 105:1530–1538. https://doi.org/10.1017/S0007114510005246
EFSA Scientific Committee (2012) Guidance on selected default values to be used by the EFSA scientific committee, scientific panels and units in the absence of actual measured data. EFSA J 10:2579. https://doi.org/10.2903/j.efsa.2012.2579
StataCorp, (2019) Stata Statistical Software: Release 16. StatCorp LLC, College Station
R Core Team (2016) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing
Cuparencu C, Praticó G, Hemeryck LY, Sri Harsha PSC, Noerman S, Rombouts C, Xi M, Vanhaecke L, Hanhineva K, Brennan L, Dragsted LO (2019) Biomarkers of meat and seafood intake: an extensive literature review. Genes Nutr. https://doi.org/10.1186/s12263-019-0656-4
Arterburn LM, Hall EB, Oken H (2006) Distribution, interconversion, and dose response of n−3 fatty acids in humans. Am J Clin Nutr 83:1467–1476. https://doi.org/10.1093/ajcn/83.6.1467S
Bratlie M, Hagen IV, Helland A, Midttun Ø, Ulvik A, Rosenlund G, Sveier H, Mellgren G, Ueland PM, Gudbrandsen OA (2020) Five salmon dinners per week were not sufficient to prevent the reduction in serum vitamin D in autumn at 60° north latitude: a randomised trial. Br J Nutr 123:419–427. https://doi.org/10.1017/s0007114519002964
Helland A, Bratlie M, Hagen IV, Midttun Ø, Sveier H, Mellgren G, Ueland PM, Gudbrandsen OA (2021) Effect of high intake of cod or salmon on serum total neopterin concentration: a randomised clinical trial. Eur J Nutr 60:3237–3248. https://doi.org/10.1007/s00394-021-02497-0
Fairweather-Tait SJ, Collings R, Hurst R (2010) Selenium bioavailability: current knowledge and future research requirements. Am J Clin Nutr 91:1484–1491. https://doi.org/10.3945/ajcn.2010.28674J
Holmquist E, Brantsæter AL, Meltzer HM, Jacobsson B, Barman M, Sengpiel V (2021) Maternal selenium intake and selenium status during pregnancy in relation to preeclampsia and pregnancy-induced hypertension in a large Norwegian Pregnancy Cohort Study. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.149271
van Asselt DZ, de Groot LC, van Staveren WA, Blom HJ, Wevers RA, Biemond I, Hoefnagels WH (1998) Role of cobalamin intake and atrophic gastritis in mild cobalamin deficiency in older Dutch subjects. Am J Clin Nutr 68:328–334. https://doi.org/10.1093/ajcn/68.2.328
Stoffaneller R, Morse NL (2015) A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients 7:1494–1537. https://doi.org/10.3390/nu7031494
Saunders CM, Rehbinder EM, Carlsen KCL, Gudbrandsgard M, Carlsen KH, Haugen G, Hedlin G, Jonassen CM, Sjøborg KD, Landrø L, Nordlund B, Rudi K, SkjervenSöderhällStaffVettukattilCarlsen OHCACRMH (2019) Food and nutrient intake and adherence to dietary recommendations during pregnancy: a Nordic mother-child population-based cohort. Food Nutr Res. https://doi.org/10.29219/fnr.v63.3676
Solvik BS, Øyen J, Kvestad I, Markhus MW, Ueland PM, McCann A, Strand TA (2021) Biomarkers and fatty fish intake: a randomized controlled trial in Norwegian Preschool Children. J Nutr 151:2134–2141. https://doi.org/10.1093/jn/nxab112
VKM (2014) Benefit-risk assessment of fish and fish products in the Norwegian diet - an update. Scientific Opinion of the Scientific Steering Committee, Oslo
Mozaffarian D, Rimm EB (2006) Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 296:1885–1899. https://doi.org/10.1001/jama.296.15.1885
Rahman MM, Hossain KFB, Banik S, Sikder MT, Akter M, Bondad SEC, Rahaman MS, Hosokawa T, Saito T, Kurasaki M (2019) Selenium and zinc protections against metal-(loids)-induced toxicity and disease manifestations: a review. Ecotoxicol Environ Saf 168:146–163. https://doi.org/10.1016/j.ecoenv.2018.10.054
Bastos SC, Tavares T, de SousaGomesPimenta ME, Leal R, Fabrício LF, Pimenta CJ, Nunes CA, Pinheiro AC (2014) Fish filleting residues for enrichment of wheat bread: chemical and sensory characteristics. J Food Sci Technol 51:2240–2245. https://doi.org/10.1007/s13197-014-1258-1
Acknowledgements
The authors gratefully acknowledge the participants who volunteered to this study. We would like to thank Amanda Rundblad, Jūratė Šaltytė Benth, Knut Tomas Dalen and Nina Gunnes for their contribution in the conduct of the project.
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital). The study received financial support from the University of Oslo and FHF–Norwegian Seafood Research Fund, Oslo, Norway.
Author information
Authors and Affiliations
Contributions
KSH, IO, TS, SMU and KBH conceived and designed the study; KSH and IO conducted the study; KSH, IO, TO, SU and KBH performed the statistical analyses and interpreted the results; KSH, SMU and KBH wrote the manuscript and had primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
During the past 5 years, SMU has received research grants from Mills DA, TINE BA and Olympic Seafood, none of which are related to the content of this manuscript. During the past 5 years, KBH has received research grants or honoraria from Mills DA, TINE BA, Olympic Seafood, Amgen, Sanofi and Pronova, none of which are related to the content of this manuscript. KSH, IO, TO and TS have no conflicts of interest.
Ethics approval
The study was registered at ClinicalTrials.gov (ClinicalTrials.gov Identifier: NCT03764423) and conducted according to the guidelines laid down in the Declaration of Helsinki. The Regional Ethics Committee for Medical Research in South East Norway approved the study.
Consent to participate
All participants gave their written informed consent.
Consent for publication
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hustad, K.S., Ottestad, I., Olsen, T. et al. Salmon fish protein supplement increases serum vitamin B12 and selenium concentrations: secondary analysis of a randomised controlled trial. Eur J Nutr 61, 3085–3093 (2022). https://doi.org/10.1007/s00394-022-02857-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02857-4