Abstract
Purpose
Soy whey is a byproduct generated from the processing of several soybean products. Its valorization has continued to attract significant research interest in recent times due to the nutritional and bioactive potency of its chemical composition. Herein, the neuroprotective potency of a soy whey fermented by Cordyceps militaris SN-18 against hydrogen peroxide (H2O2)-induced oxidative injury in PC12 cells was investigated.
Methods
The phenolic compositions were analyzed by high-performance liquid chromatography. Antioxidant activities were assessed by ABTS•+ scavenging assay, DPPH radical scavenging assay, reducing power assay, and ferric reducing antioxidant power assay. The neuroprotective effects of fermented soy whey (FSW) were investigated based on the oxidative injury model in PC12 cells.
Results
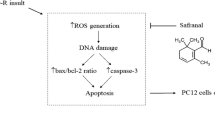
FSW possessed higher total phenolic content and antioxidant activities compared with unfermented soy whey (UFSW) and that most of the isoflavone glycosides were hydrolyzed into their corresponding aglycones during fermentation. The extract from FSW exhibited a greater protective effect on PC12 cells against oxidative injury by promoting cell proliferation, restoring cell morphology, inhibiting lactic dehydrogenase leakage, reducing reactive oxygen species levels, and enhancing antioxidant enzyme activities compared with that from UFSW. Additionally, cell apoptosis was significantly inhibited by FSW through down-regulation of caspase-3, caspase-9, and Bax and up-regulation of Bcl-2 and Bcl-xL. S-phase cell arrest was attenuated by FSW through increasing cyclin A, CDK1 and CDK2, and decreasing p21 protein.
Conclusion
Fermentation with C. militaris SN-18 could significantly improve the bioactivity of soy whey by enhancing the ability of nerve cells to resist oxidative damage.






Similar content being viewed by others
Abbreviations
- HPLC:
-
High-performance liquid chromatography
- UFSW:
-
Unfermented soy whey
- FSW:
-
Fermented soy whey
- H2O2 :
-
Hydrogen peroxide
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ABTS:
-
2,2-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
- FRAP:
-
Ferric reducing antioxidant power
- RP:
-
Reducing powder
- TPC:
-
Total phenolic content
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- FBS:
-
Fetal bovine serum
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- LDH:
-
Lactate dehydrogenase
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- GSH-Px:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- ROS:
-
Reactive oxygen species
- GAE:
-
Gallic acid equivalent
- NBT:
-
Nitro blue tetrazolium
- PI:
-
Prodium iodide
References
Fung W, Liong M (2010) Evaluation of proteolytic and ACE-inhibitory activity of Lactobacillus acidophilus in soy whey growth medium via response surface methodology. LWT-Food Sci Technol 43:563–567
Azi F, Tu C, Rasheed HA, Dong M (2020) Comparative study of the phenolics, antioxidant, and metagenomic composition of novel soy whey-based beverages produced using three different water kefir microbiota. Int J Food Sci Technol 55:1689–1697
Tu C, Tang S, Azi F, Hu W, Dong M (2019) Use of kombucha consortium to transform soy whey into a novel functional beverage. J Funct Foods 52:81–89
Fei Y, Liu L, Liu D, Chen L, Tan B, Fu L, Li L (2017) Investigation on the safety of Lactobacillus amylolyticus L6 and its fermentation properties of tofu whey. LWT-Food Sci Technol 84:314–322
Mitra D, Pometto AL, Khanal SK, Karki B, Brehmstecher BF, Van Leeuwen J (2010) Value-added production of nisin from soy whey. Appl Biochem Biotechnol 162:1819–1833
Li C, Rui X, Zhang Y, Cai F, Chen X, Jiang M (2017) Production of tofu by lactic acid bacteria isolated from naturally fermented soy whey and evaluation of its quality. LWT-Food Sci Technol 82:227–234
Xiao Y, Wang L, Rui X, Li W, Chen X, Jiang M, Dong M (2015) Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1–6. J Funct Foods 12:33–44
Zhang J, Wen C, Duan Y, Zhang H, Ma H (2019) Advance in Cordyceps militaris (Linn) Link polysaccharides: isolation, structure, and bioactivities: a review. Int J Biological Macromol 132:906–914
Manoharan S, Guillemin GJ, Abiramasundari RS, Essa MM, Akbar M, Akbar MD (2016) The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkison’s disease, and Huntington’s disease: a mini review. Oxid Med Cell Longev 2016:8590578
Shih PH, Chan YC, Liao JW, Wang MF, Yen GC (2010) Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J Nutr Biochem 21:598–605
Butterfield DA, Boydkimball D (2018) Oxidative stress, amyloid-β peptide, and altered key molecular pathways in the pathogenesis and progression of Alzheimer’s disease. J Alzheimers Dis 62:1345–1367
Cheignon C, Tomas M, Bonnefontrousselot D, Faller P, Hureau C, Collin F (2018) Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol 14:450–464
Peng S, Zhang B, Yao J, Duan D, Fang J (2015) Dual protection of hydroxytyrosol, an olive oil polyphenol, against oxidative damage in PC12 cells. Food Funct 6:2091–2100
Huang X, Zhen J, Dong S, Zhang H, Van Halmlutterodt N, Yuan L (2019) DHA and vitamin E antagonized the Aβ25–35-mediated neuron oxidative damage through activation of Nrf2 signaling pathways and regulation of CD36, SRB1 and FABP5 expression in PC12 cells. Food Funct 10:1049–1061
Li M, Tao X, Fei Z, Wang M, Song H, Xing X, Lu B (2018) Neuroprotective effects of four phenylethanoid glycosides on H2O2-induced apoptosis on PC12 cells via the Nrf2/ARE pathway. Int J Mol Sci 19:1135
Hansen RA, Gartlehner G, Webb AP et al (2008) Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging 3(2):211–225
Mimica N, Presecki P (2009) Side effects of approved antidementives. Psychiatr Danub 21(1):108–113
Choi WY, Kang DH, Lee HY (2018) Enhancement of neuroprotective effects of spirulina maxima by a low-temperature extraction process with ultrasonic pretreatment. Biotechnol Bioprocess Eng 23:415–423
Crispo JAG, Ansell DR, Piche M, Eibl JK, Khaper N, Ross GM, Tai TCTC (2010) Protective effects of polyphenolic compounds on oxidative stress-induced cytotoxicity in PC12 cells. Can J Physiol Pharmcol 88:429–438
Cho CH, Jang H, Lee M, Kang H, Heo HJ, Kim D (2017) Sea buckthorn (Hippophae rhamnoides L.) leaf extracts protect neuronal PC12 cells from oxidative stress. J Microbiol Biotechnol 27:1257–1265
Im S, Yoon H, Nam TG, Heo HJ, Lee CY, Kim D (2010) Antineurodegenerative effect of phenolic extracts and caffeic acid derivatives in romaine lettuce on neuron-like PC12 cells. J Med Food 13:779–784
Levites Y, Amit T, Youdim MBH, Mandel S (2002) Involvement of protein kinase C activation and cell survival/ cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem 277:30574–30580
Tang J, Yan Y, Ran L, Mi J, Sun Y, Lu L, Gao Y, Zeng X, Cao Y (2017) Isolation, antioxidant property and protective effect on PC12 cell of the main anthocyanin in fruit of Lycium ruthenicum Murray. J Funct Foods 30:97–107
Sonee M, Sum T, Wang C, Mukherjee SK (2004) The soy isoflavone, genistein, protects human cortical neuronal cells from oxidative stress. Neurotoxicology 25:885–891
Qian Y, Guan T, Huang M et al (2012) Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-κB activation in a cerebral ischemia mouse model. Neurochem Int 60:759–767
Yoon G, Park S (2014) Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr Res Pract 8:618–624
Zhang B, Li W, Dong M (2017) Flavonoids of kudzu root fermented by Eurtotium cristatum protected rat pheochromocytoma line 12 (PC12) cells against H2O2-induced apoptosis. Int J Mol Sci 18:2754
Xiao Y, Xing G, Rui X, Li W, Chen X, Jiang M, Dong M (2014) Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J Funct Foods 10:210–222
Zhang Y, Yin L, Huang L, Tekliye M, Xia X, Li J, Dong M (2020) Composition, antioxidant activity, and neuroprotective effects of anthocyanin-rich extract from purple highland barley bran and its promotion on autophagy. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127849
Li P, Ma K, Wu H, Wu Y, Li B (2017) Isoflavones induce BEX2-dependent autophagy to prevent ATR-induced neurotoxicity in SH-SY5Y cells. Cell Physiol Biochem 43:1866–1879
Soccol CR, Costa ESFD, Letti LAJ, Karp SG, Woiciechowski AL, Vandenberghe LPDS (2017) Recent developments and innovations in solid state fermentation. Biotech Res Innov 1:52–71
Acostaestrada BA, Gutierrezuribe JA, Sernasaldivar SO (2014) Bound phenolics in foods, a review. Food Chem 152:46–55
Wang L, Luo Y, Wu Y, Liu Y, Wu Z (2018) Fermentation and complex enzyme hydrolysis for improving the total soluble phenolic contents, flavonoid aglycones contents and bio-activities of guava leaves tea. Food Chem 264:189–198
Bei Q, Chen G, Lu F, Wu S, Wu Z (2018) Enzymatic action mechanism of phenolic mobilization in oats (Avena sativa L.) during solid-state fermentation with Monascus anka. Food Chem 245:297–304
Vong WC, Hua XY, Liu S (2018) Solid-state fermentation with Rhizopus oligosporus and Yarrowia lipolytica improved nutritional and flavour properties of okara. LWT-Food Sci Technol 90:316–322
Santos VAQ, Nascimento CG, Schmidt CAP, Mantovani D, Dekker RFH, Cunha MAAD (2018) Solid-state fermentation of soybean okara: isoflavones biotransformation, antioxidant activity and enhancement of nutritional quality. LWT-Food Sci Technol 92:509–515
Naim M, Gestetner B, Bondi A, Birk Y (1976) Antioxidative and antihemolytic activities of soybean isoflavones. J Agric Food Chem 24:1174–1177
Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M (2000) Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr 130:1695–1699
Xia X, Dai Y, Wu H, Liu X, Wang Y, Yin L, Wang Z, Li X, Zhou J (2019) Kombucha fermentation enhances the health-promoting properties of soymilk beverage. J Funct Foods. https://doi.org/10.1016/j.jff.2019.103549
Man AWC, Li H, Xia N (2020) Impact of lifestyles (diet and exercise) on vascular health: oxidative stress and endothelial function. Oxid Med Cell Longev 2020:1496462
Tan BL, Norhaizan ME (2019) Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 11(11):2579
Durazzo TC, Mattsson N, Weiner MW (2014) Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimers Dement 10:S122–S145
Sonee M, Sum T, Wang C, Mukherjee SK (2004) The soy isoflavone, genistein, protects human cortical neuronal cells from oxidative stress. Neurotoxicology 25(5):885–891
Xu JX, Song HP, Bu QX, Feng DP, Xu XF, Sun QR, Li XL (2015) Isoflavone attenuates the caspase-1 and caspase-3 level in cell model of Parkinsonism. Behav Neurol. https://doi.org/10.1155/2015/725897
Miura YH, Tomita I, Watanabe T, Hirayama T, Fukui S (1998) Active oxygens generation by flavonoids. Biol Pharm Bull 21(2):93–96
Chen B, Yue R, Yang Y, Zeng H, Chang W, Gao N, Yuan X, Zhang W, Shan L (2015) Protective effects of (E)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeine against hydrogen peroxide-induced injury in PC12 cells. Neurochem Res 40(3):531–541
Sharma GP, Gurung SK, Inam A, Nigam L, Bist A, Mohapatra D, Senapati S, Subbarao N, Azam A, Mondal N (2019) CID-6033590 inhibits p38MAPK pathway and induces S-phase cell cycle arrest and apoptosis in DU145 and PC-3 cells. Toxicol In Vitro 60:420–436
Zaheer K, Humayoun Akhtar M (2017) An updated review of dietary isoflavones: nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr 57(6):1280–1293
Acknowledgements
This work was financially supported by the Science and Technology Program of Jiangsu Province (BE2019355), and the National Natural Science Foundation of China (Grant No.31501460).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yin, L., Zhang, Y., Wang, L. et al. Neuroprotective potency of a soy whey fermented by Cordyceps militaris SN-18 against hydrogen peroxide-induced oxidative injury in PC12 cells. Eur J Nutr 61, 779–792 (2022). https://doi.org/10.1007/s00394-021-02679-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02679-w