Abstract
Purpose
Several studies have demonstrated the properties of hydroxytyrosol, a phenolic compound present in olive oils and olives with a well-characterized impact on human health. Nevertheless, some knowledge gaps remain on its bioavailability and metabolism; overall concerning to the real rate per cent of absorption and biovailability of dietary hydroxytyrosol and the influence of the dietary food-containing hydroxytyrosol on it.
Methods
A double-blind study was performed including 20 volunteers who ingested 5 mg of hydroxytyrosol through diverse food matrices, to discover the influence on pharmacokinetics and bioavailability of HT metabolites (hydroxytyrosol acetate, 3,4-dihydroxyphenylacetic acid (DOPAC), tyrosol, and homovanillic alcohol) of the distinct matrices by UHPLC–ESI–QqQ–MS/MS.
Results
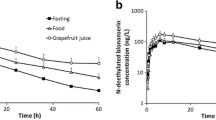
The HT pharmacokinetics after consumption of different food matrices was strongly dependent on the food matrix. In this aspect, the intake of extra virgin olive exhibited significantly higher plasma concentrations after 30 min of oral intake (3.79 ng/mL) relative to the control. Regarding the hydroxytyrosol bioavailability, the intake of extra virgin olive oil, as well as fortified refined olive, flax, and grapeseed oils provided significantly higher urinary contents (0.86, 0.63, 0.55, and 0.33 µg/mg creatinine, respectively) compared with basal urine, whereas hydroxytyrosol metabolites showed no significant changes. No differences were found between men and women.
Conclusions
The metabolic profile of hydroxytyrosol is influenced by the food matrix in which is incorporated, with the oily nature for the final bioavailability being relevant. Extra virgin olive oil was identified as the best matrix for this compound. The results described contribute to the understanding of the relevance of the food matrices for the final absorption of hydroxytyrosol and hence, the achievement of the highest health protection potential.




Similar content being viewed by others
Abbreviations
- Amu:
-
Atomic mass unit
- DOPAC:
-
3,4-Dihydroxyphenylacetic acid
- HT:
-
Hydroxytyrosol
- HTA:
-
Hydroxytyrosol acetate
- HValc:
-
Homovanillic alcohol
- MRM:
-
Multiple reaction monitoring
- SPE:
-
Solid phase extraction
- Tyr:
-
Tyrosol
- UHPLC–ESI–QqQ–MS/MS:
-
Ultra-high performance liquid chromatography coupled to electrospray ionization and triple quadrupole mass spectrometry
References
Hoffman R, Gerber M (2015) Food processing and the mediterranean diet. Nutrients 7:7925–7964. https://doi.org/10.3390/nu7095371
Hazas López de las MC, Piñol C, Macià A et al (2016) Differential absorption and metabolism of hydroxytyrosol and its precursors oleuropein and secoiridoids. J Funct Foods 22:52–63. https://doi.org/10.1016/j.jff.2016.01.030
Vilaplana-Pérez C, Auñón D, García-Flores LA, Gil-Izquierdo A (2014) Hydroxytyrosol and potential uses in cardiovascular diseases, cancer, and AIDS. Front Nutr 1:18. https://doi.org/10.3389/fnut.2014.00018
Angeloni C, Malaguti M, Barbalace MC, Hrelia S (2017) Bioactivity of olive oil phenols in neuroprotection. Int J Mol Sci 18:2230. https://doi.org/10.3390/ijms18112230
Peyrol J, Riva C, Amiot MJ (2017) Hydroxytyrosol in the prevention of the metabolic syndrome and related disorders. Nutrients. https://doi.org/10.3390/nu9030306
Robles-Almazan M, Pulido-Moran M, Moreno-Fernández J et al (2018) Hydroxytyrosol: bioavailability, toxicity, and clinical applications. Food Res Int 105:654–667. https://doi.org/10.1016/j.foodres.2017.11.053
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2011) Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J 9(4):2033. https://doi.org/10.2903/j.efsa.2011.2033
Segura M, Chel L, Betancur D (2018) Efecto de la digestión en la biodisponibilidad de péptidos con actividad biológica. Rev Child Nutr 37(3):386–391. https://doi.org/10.4067/S0717-75182010000300014
Lafay S, Gil-Izquierdo A (2008) Bioavailability of phenolic acids. Phytochem Rev 7:301–311. https://doi.org/10.1007/s11101-007-9077-x
Domínguez-Perles R, Auñón D, Ferreres F, Gil-Izquierdo A (2017) Gender differences in plasma and urine metabolites from Sprague-Dawley rats after oral administration of normal and high doses of hydroxytyrosol, hydroxytyrosol acetate, and DOPAC. Eur J Nutr. https://doi.org/10.1007/s00394-015-1071-2
Miró-Casas E, Covas MI, Farré M et al (2003) Hydroxytyrosol disposition in human. Clin Chem 49:945–952. https://doi.org/10.1373/49.6.945
Bohn T (2014) Dietary factors affecting polyphenol bioavailability. Nutr Rev 72:429–452. https://doi.org/10.1111/nure.12114
Gouvinhas I, Machado N, Sobreira C, Domínguez-Perles R, Gomes S, Rosa E, Barros A (2017) Critical review on the significance of Olive phytochemicals in plant physiology and human health. Molecules. https://doi.org/10.3390/molecules22111986
Suárez M, Valls RM, Romero MP, Macià A, Fernández S, Giralt M, Solà R, Motilva MJ (2011) Bioavailability of phenols from a phenol-enriched olive oil. Br J Nutr 106:1691–1701. https://doi.org/10.1017/S0007114511002200
Mateos R, Martinez-Lopez S, Baeza Arevalo G, Amigo-Benavent M, Sarria B, Bravo-Clemente L (2016) Hydroxytyrosol in functional hydroxytyrosol-enriched biscuits is highly bioavailable and decreases oxidised low density lipoprotein levels in humans. Food Chem 205:248–256. https://doi.org/10.1016/j.foodchem.2016.03.011
Domínguez-Perles R, Auñón D, Ferreres F, Gil-Izquierdo A (2017) Physiological linkage of gender, bioavailable hydroxytyrosol derivatives, and their metabolites with systemic catecholamine metabolism. Food Funct. https://doi.org/10.1039/c7fo01124e
Pastor A, Rodríguez-Morato J, Olesti E, Pujadas M, Pérez-Mana C, Khymenets O, Fito M, Covas MI, Solà R, Motilva MJ, Farrè M, de la Torre R (2016) Analysis of free hydroxytyrosol in human plasma following the administration of olive oil. J Chromatogr A 1437:183–190. https://doi.org/10.1016/j.chroma.2016.02.016
de Bock M, Thorstensen EB, Derraik JGB, Henderson HV, Hofman PL, Cutfield WS (2013) Human absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extract. Mol Nutr Food Res 57:2079–2085. https://doi.org/10.1002/mnfr.201200795
Ruiz-García A, Bermejo M, Moss A, Casabo VG (2008) Pharmacokinetics in drug discovery. J Pharm Sci 97:654–690. https://doi.org/10.1002/jps.21009
Amiot M (2014) Olive oil and health effects: From epidemiological studies to the molecular mechanisms of phenolic fraction. OCL 21:D512. https://doi.org/10.1051/ocl/2014029
Parkinson L, Cicerale S (2016) The health benefiting mechanisms of virgin olive oil phenolic compounds. Molecules. https://doi.org/10.3390/molecules21121734
Chung TDY, Terry DB, Smith LH (2004) In vitro and in vivo assessment of adme and pk properties during lead selection and lead optimization guidelines, benchmarks and rules of thumb. In: Sittampalam GS, Grossman A, Brimacombe K, et al. (eds) Assay guidance manual. Eli Lilly & Company and the National Center for Advancing Translational Sciences, Bethesda
D’Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R (2010) Bioavailability of the polyphenols: status and controversies. Int J Mol Sci 11:1321–1342. https://doi.org/10.3390/ijms11041321
de la Torre R (2008) Bioavailability of olive oil phenolic compounds in humans. Inflammopharmacology 16:245–247. https://doi.org/10.1007/s10787-008-8029-4
González-Santiago M, Fonolla J, López-Huertas E (2010) Human absorption of a supplement containing purified hydroxytyrosol, a natural antioxidant from olive oil, and evidence for its transient association with low-density lipoproteins. Pharmacol Res 61:364–370. https://doi.org/10.1016/j.phrs.2009.12.016
Visioli F, Galli C, Bornet F, Mattei A, Patelli R, Galli G, Caruso D (2000) Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett 468:159–160. https://doi.org/10.1016/s0014-5793(00)01216-3
Visioli F, Galli C, Grande S, Colonnelli K, Patelli C, Galli G, Caruso D (2003) Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J Nutr 133:2612–2615. https://doi.org/10.1093/jn/133.8.2612
D’Angelo S, Manna C, Migliardi V, Mazzoni O, Morrica P, Capasso G, Pontoni G, Galletti P, Zappia V (2001) Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab Dispos 29:1492–1498
Vlavcheski F, Young M, Tsiani E (2019) Antidiabetic Effects of Hydroxytyrosol In Vitro and In Vivo Evidence. Antioxidants (Basel, Switzerland) 8:188. https://doi.org/10.3390/antiox8060188
Campesi I, Marino M, Cipolletti M, Romani A, Franconi F (2018) Put “gender glasses” on the effects of phenolic compounds on cardiovascular function and diseases. Eur J Nutr 57:2677–2691. https://doi.org/10.1007/s00394-018-1695-0
Murtaza G, Ullah N, Mukhtar F et al (2017) Phytotherapeutics: the emerging role of intestinal and hepatocellular transporters in drug interactions with botanical supplements. Molecules. https://doi.org/10.3390/molecules22101699
Weinbrenner T, Fitó M, de la Torre R, Nawazish S, Muneer S (2004) Olive oils high in phenolic compounds modulate oxidative/antioxidative status in men. J Nutr 134:2314–2321. https://doi.org/10.1093/jn/134.9.2314
Acknowledgements
This work was partially funded by the “Fundación Séneca de la Región de Murcia” Grupo de Excelencia 19900/GERM/15, the Spanish project AGL2017-83386-R from the Spanish Ministry of Science, Innovation and Universities. R.D.P. was sponsored by a Postdoctoral Contract (Juan de la Cierva de Incorporación ICJI-2015-25373) from the Ministry of Economy, Industry and Competitiveness of Spain. The authors thank the English expert reviewer (Mario G. Fon, MS) for the revision of the English style and grammar.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Alemán-Jiménez, C., Domínguez-Perles, R., Medina, S. et al. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food matrix in humans. Eur J Nutr 60, 905–915 (2021). https://doi.org/10.1007/s00394-020-02295-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02295-0