Abstract
Purpose
We examined the effect on the postprandial plasma metabolome of protein pre-meals before a fat-rich main meal.
Methods
Two randomized, cross-over meal studies were conducted to test the dose–response effect (0 g, 10 g, 20 g) of a pre-meal with whey protein (WP) (PREMEAL I), and the effect of protein quality (10 g WP, casein, or gluten) and timing (− 15 min vs − 30 min) of the pre-meal (PREMEAL II). Participants with metabolic syndrome received one of the test meals on each test day, − 15 min (or − 30 min) prior to a standardized fat-rich breakfast. Plasma samples were collected at − 15 min (or − 30 min), 0, 120, 240 a nd 360 min and analyzed using liquid chromatography–mass spectrometry with an untargeted method.
Results
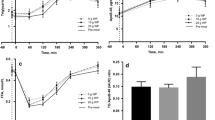
Pre-meal WP intake elevated plasma branched-chain amino acids (BCAA), aromatic amino acids and methionine and decreased plasma LPC (16:0) and PC (32:1) levels before the main meal. Early (− 15 to 0 min) aromatic amino acids and BCAA in response to pre-meal WP partially predict the glucose and insulin response after the main meal. A pre-meal with WP altered the postprandial plasma metabolic pattern of acyl-carnitines, specific PCs, LPCs and LPEs, betaine, citric acid, linoleic acid, and β-hydroxypalmitic acid compared to no pre-meal. The casein and WP pre-meals exhibited similar postprandial amino acid responses whereas a pre-meal with gluten resulted in lower levels of plasma amino acids and its metabolites.
Conclusion
A pre-meal with protein affects the postprandial metabolic pattern indicating facilitated glucose and lipid disposal from plasma in participants with metabolic syndrome.




Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the curve
- CDE:
-
Collision dissociation energies
- CVDs:
-
Cardiovascular diseases
- ESI:
-
Electrospray ionization
- FFAs:
-
Free fatty acids
- GLP-1:
-
Glucagon-like peptide-1
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- LC/MS:
-
Liquid chromatography/mass spectrometry
- LPCs:
-
Lysophosphatidylcholines
- LPEs:
-
Lysophosphatidylethanolamines
- m/z:
-
Mass to charge ratio
- MetS:
-
Metabolic syndrome
- MS/MS:
-
Tandem mass spectrometry
- MS:
-
Mass spectrometry
- PCA:
-
Principal component analysis
- PCs:
-
Phosphatidylcholines
- PLS-DA:
-
Partial least squares discriminant analysis
- PYY:
-
Peptide YY
- q-TOF–MS:
-
Quadrupole-time of flight mass spectrometer
- ROC:
-
Area under the receiver-operator curve
- RT:
-
Retention time
- T2DM:
-
Type 2 diabetes mellitus
- UPLC:
-
Ultra-performance liquid chromatography
- VIP:
-
Variable importance in projection
- WP:
-
Whey protein
References
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120(16):1640–1645. https://doi.org/10.1161/circulationaha.109.192644
Samson SL, Garber AJ (2014) Metabolic syndrome. Endocrinol Metab Clin North Am 43(1):1–23. https://doi.org/10.1016/j.ecl.2013.09.009
Dunkley AJ, Charles K, Gray LJ, Camosso-Stefinovic J, Davies MJ, Khunti K (2012) Effectiveness of interventions for reducing diabetes and cardiovascular disease risk in people with metabolic syndrome: systematic review and mixed treatment comparison meta-analysis. Diabetes Obes Metab 14(7):616–625. https://doi.org/10.1111/j.1463-1326.2012.01571.x
Bonuccelli S, Muscelli E, Gastaldelli A, Barsotti E, Astiarraga BD, Holst JJ, Mari A, Ferrannini E (2009) Improved tolerance to sequential glucose loading (Staub-Traugott effect): size and mechanisms. Am J Physiol Endocrinol Metab 297(2):E532–E537. https://doi.org/10.1152/ajpendo.00127.2009
Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH (2010) Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr 91(4):966–975. https://doi.org/10.3945/ajcn.2009.28406
Allerton DM, Rumbold PLS, West DJ, Stevenson EJ (2019) Effect of supplemental whey protein timing on postprandial glycaemia in centrally obese males. Br J Nutr 121(6):637–646. https://doi.org/10.1017/S0007114518003793
Akhavan T, Luhovyy BL, Panahi S, Kubant R, Brown PH, Anderson GH (2014) Mechanism of action of pre-meal consumption of whey protein on glycemic control in young adults. J Nutr Biochem 25(1):36–43. https://doi.org/10.1016/j.jnutbio.2013.08.012
Gonzalez JT (2014) Paradoxical second-meal phenomenon in the acute postexercise period. Nutrition (Burbank, Los Angeles County, Calif) 30(9):961–967. https://doi.org/10.1016/j.nut.2013.12.001
Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM (2004) Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr 80(5):1246–1253. https://doi.org/10.1093/ajcn/80.5.1246
Holmer-Jensen J, Mortensen LS, Astrup A, de Vrese M, Holst JJ, Thomsen C, Hermansen K (2013) Acute differential effects of dietary protein quality on postprandial lipemia in obese non-diabetic subjects. Nutr Res (New York, NY) 33(1):34–40. https://doi.org/10.1016/j.nutres.2012.11.004
Dudzik D, Barbas-Bernardos C, Garcia A, Barbas C (2018) Quality assurance procedures for mass spectrometry untargeted metabolomics. A review. J Pharm Biomed Anal 147:149–173. https://doi.org/10.1016/j.jpba.2017.07.044
Bjornshave A, Holst JJ, Hermansen K (2018) A pre-meal of whey proteins induces differential effects on glucose and lipid metabolism in subjects with the metabolic syndrome: a randomised cross-over trial. Eur J Nutr. https://doi.org/10.1007/s00394-018-1684-3
Bjornshave A, Johansen TN, Amer B, Dalsgaard TK, Holst JJ, Hermansen K (2019) Pre-meal and postprandial lipaemia in subjects with the metabolic syndrome: effects of timing and protein quality (randomised crossover trial). Br J Nutr. https://doi.org/10.1017/s0007114518003264
Barri T, Holmer-Jensen J, Hermansen K, Dragsted LO (2012) Metabolic fingerprinting of high-fat plasma samples processed by centrifugation- and filtration-based protein precipitation delineates significant differences in metabolite information coverage. Anal Chim Acta 718:47–57. https://doi.org/10.1016/j.aca.2011.12.065
Pluskal T, Castillo S, Villar-Briones A, Oresic M (2010) MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform 11:395. https://doi.org/10.1186/1471-2105-11-395
Bijlsma S, Bobeldijk I, Verheij ER, Ramaker R, Kochhar S, Macdonald IA, van Ommen B, Smilde AK (2006) Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal Chem 78(2):567–574. https://doi.org/10.1021/ac051495j
Brereton RG, Lloyd GR (2014) Partial least squares discriminant analysis: taking the magic away. J Chemom 28(4):213–225. https://doi.org/10.1002/cem.2609
Chen T, Guestrin C (2016) XGBoost: A Scalable Tree Boosting System. In: Paper presented at the Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, California, USA
Bergstra J, Bengio Y (2012) Random search for hyper-parameter optimization. J Mach Learn Res 13:281–305
Castillo S, Gopalacharyulu P, Yetukuri L, Orešič M (2011) Algorithms and tools for the preprocessing of LC–MS metabolomics data. Chemom Intell Lab Syst 108(1):23–32. https://doi.org/10.1016/j.chemolab.2011.03.010
Storey JD (2002) A direct approach to false discovery rates. J R Stat Soc Ser B (Statistical Methodology) 64(3):479–498
Gurdeniz G, Rago D, Bendsen NT, Savorani F, Astrup A, Dragsted LO (2013) Effect of trans fatty acid intake on LC–MS and NMR plasma profiles. PLoS One 8(7):e69589. https://doi.org/10.1371/journal.pone.0069589
Rago D, Mette K, Gürdeniz G, Marini F, Poulsen M, Dragsted LO (2013) A LC–MS metabolomics approach to investigate the effect of raw apple intake in the rat plasma metabolome. Metabolomics 9(6):1202–1215. https://doi.org/10.1007/s11306-013-0534-9
Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TWM, Fiehn O, Goodacre R, Griffin JL, Hankemeier T, Hardy N, Harnly J, Higashi R, Kopka J, Lane AN, Lindon JC, Marriott P, Nicholls AW, Reily MD, Thaden JJ, Viant MR (2007) Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3(3):211–221. https://doi.org/10.1007/s11306-007-0082-2
King DG, Walker M, Campbell MD, Breen L, Stevenson EJ, West DJ (2018) A small dose of whey protein co-ingested with mixed-macronutrient breakfast and lunch meals improves postprandial glycemia and suppresses appetite in men with type 2 diabetes: a randomized controlled trial. Am J Clin Nutr 107(4):550–557. https://doi.org/10.1093/ajcn/nqy019
Bernard JR, Liao Y-H, Hara D, Ding Z, Chen C-Y, Nelson JL, Ivy JL (2011) An amino acid mixture improves glucose tolerance and insulin signaling in Sprague–Dawley rats. Am J Physiol Endocrinol Metab 300(4):E752–E760. https://doi.org/10.1152/ajpendo.00643.2010
Doi M, Yamaoka I, Nakayama M, Sugahara K, Yoshizawa F (2007) Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am J Physiol Endocrinol Metab 292(6):E1683–1693. https://doi.org/10.1152/ajpendo.00609.2006
Zhao X, Han Q, Liu Y, Sun C, Gang X, Wang G (2016) The relationship between branched-chain amino acid related metabolomic signature and insulin resistance: a systematic review. J Diabetes Res 2016:2794591. https://doi.org/10.1155/2016/2794591
Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrère B, Gorroochurn P, Teixeira J, Brantley PJ, Stevens VJ, Hollis JF, Appel LJ, Lien LF, Batch B, Newgard CB, Svetkey LP (2012) Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 55(2):321–330. https://doi.org/10.1007/s00125-011-2356-5
Gannon NP, Schnuck JK, Vaughan RA (2018) BCAA metabolism and insulin sensitivity—dysregulated by metabolic status? Mol Nutr Food Res 62(6):e1700756. https://doi.org/10.1002/mnfr.201700756
Inubushi T, Kamemura N, Oda M, Sakurai J, Nakaya Y, Harada N, Suenaga M, Matsunaga Y, Ishidoh K, Katunuma N (2012) l-tryptophan suppresses rise in blood glucose and preserves insulin secretion in type-2 diabetes mellitus rats. J Nutr Sci Vitaminol 58(6):415–422
Lin HV, Efanov AM, Fang X, Beavers LS, Wang X, Wang J, Gonzalez Valcarcel IC, Ma T (2016) GPR142 controls tryptophan-induced insulin and incretin hormone secretion to improve glucose metabolism. PLoS One 11(6):e0157298. https://doi.org/10.1371/journal.pone.0157298
Zeng H, Tong R, Tong W, Yang Q, Qiu M, Xiong A, Sun S, Ding L, Zhang H, Yang L, Tian J (2017) Metabolic Biomarkers for prognostic prediction of pre-diabetes: results from a longitudinal cohort study. Sci Rep 7(1):6575. https://doi.org/10.1038/s41598-017-06309-6
Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost H-G, Fritsche A, Häring H-U, Hrabě de Angelis M, Peters A, Roden M, Prehn C, Wang-Sattler R, Illig T, Schulze MB, Adamski J, Boeing H, Pischon T (2013) Identification of serum metabolites associated with risk of Type 2 diabetes using a targeted metabolomic approach. Diabetes 62(2):639
Barber MN, Risis S, Yang C, Meikle PJ, Staples M, Febbraio MA, Bruce CR (2012) Plasma lysophosphatidylcholine levels are reduced in obesity and type 2 diabetes. PLoS One 7(7):e41456. https://doi.org/10.1371/journal.pone.0041456
Wallace M, Morris C, O’Grada CM, Ryan M, Dillon ET, Coleman E, Gibney ER, Gibney MJ, Roche HM, Brennan L (2014) Relationship between the lipidome, inflammatory markers and insulin resistance. Mol BioSyst 10(6):1586–1595. https://doi.org/10.1039/c3mb70529c
Kim JY, Park JY, Kim OY, Ham BM, Kim H-J, Kwon DY, Jang Y, Lee JH (2010) Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC–Q-TOF MS). J Proteome Res 9(9):4368–4375. https://doi.org/10.1021/pr100101p
Schmitz G, Ruebsaamen K (2010) Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis 208(1):10–18. https://doi.org/10.1016/j.atherosclerosis.2009.05.029
Klingler C, Zhao X, Adhikary T, Li J, Xu G, Haring HU, Schleicher E, Lehmann R, Weigert C (2016) Lysophosphatidylcholines activate PPARdelta and protect human skeletal muscle cells from lipotoxicity. Biochimica et Biophysica Acta 1861 12 Pt A:1980–1992. https://doi.org/10.1016/j.bbalip.2016.09.020
Schooneman MG, Vaz FM, Houten SM, Soeters MR (2013) Acylcarnitines: reflecting or inflicting insulin resistance? Diabetes 62(1):1–8. https://doi.org/10.2337/db12-0466
Rago D, Gürdeniz G, Ravn-Haren G, Dragsted LO (2015) An explorative study of the effect of apple and apple products on the human plasma metabolome investigated by LC–MS profiling. Metabolomics 11(1):27–39. https://doi.org/10.1007/s11306-014-0668-4
Stanstrup J, Rasmussen JE, Ritz C, Holmer-Jensen J, Hermansen K, Dragsted LO (2014) Intakes of whey protein hydrolysate and whole whey proteins are discriminated by LC–MS metabolomics. Metabolomics 10(4):719–736. https://doi.org/10.1007/s11306-013-0607-9
Giesbertz P, Ecker J, Haag A, Spanier B, Daniel H (2015) An LC–MS/MS method to quantify acylcarnitine species including isomeric and odd-numbered forms in plasma and tissues. J Lipid Res 56(10):2029–2039. https://doi.org/10.1194/jlr.D061721
Martins MB, Carvalho I (2007) Diketopiperazines: biological activity and synthesis. Tetrahedron 63(40):9923–9932. https://doi.org/10.1016/j.tet.2007.04.105
He T, Giuseppin ML (2014) Slow and fast dietary proteins differentially modulate postprandial metabolism. Int J Food Sci Nutr 65(3):386–390. https://doi.org/10.3109/09637486.2013.866639
Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM (2009) Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (Bethesda, Md: 1985) 107(3):987–992. https://doi.org/10.1152/japplphysiol.00076.2009
Stanstrup J, Schou SS, Holmer-Jensen J, Hermansen K, Dragsted LO (2014) Whey protein delays gastric emptying and suppresses plasma fatty acids and their metabolites compared to casein, gluten, and fish protein. J Proteome Res 13(5):2396–2408. https://doi.org/10.1021/pr401214w
Mihas C, Kolovou GD, Mikhailidis DP, Kovar J, Lairon D, Nordestgaard BG, Ooi TC, Perez-Martinez P, Bilianou H, Anagnostopoulou K, Panotopoulos G (2011) Diagnostic value of postprandial triglyceride testing in healthy subjects: a meta-analysis. Curr Vasc Pharmacol 9(3):271–280
Gunnerud UJ, Heinzle C, Holst JJ, Ostman EM, Bjorck IM (2012) Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS One 7(9):e44731. https://doi.org/10.1371/journal.pone.0044731
Acknowledgements
AB and KH planned and conducted the intervention studies. CTP conducted the metabolomics workflow and data analysis and drafted the manuscript. GP and LOD contributed to data analysis, identification and interpretation. LOD, CTP, AB, GP, and KH reviewed the manuscript. All the authors have read and approved the final version.
Funding
This work was supported by grants from the Danish Dairy Research Foundation and the Innovation Fund—MERITS (4105-00002B). CTP was supported by a Ph.D. grant from the Department of Nutrition, Exercise and Sports, University of Copenhagen and Hacettepe University. AB was supported by research grants from The Danish Diabetes Academy supported by the Novo Nordisk Foundation, Aarhus University and The Research Foundation of the Department of Endocrinology and Internal Medicine, Aarhus University Hospital. Protein powder was kindly provided by Arla Foods Ingredients Group P/S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CTP, GP, KH and LOD declare no conflicts of interest. AB has after termination of the study been employed at Arla Foods Ingredients Group P/S.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pekmez, C.T., Bjørnshave, A., Pratico, G. et al. Pre-meal protein intake alters postprandial plasma metabolome in subjects with metabolic syndrome. Eur J Nutr 59, 1881–1894 (2020). https://doi.org/10.1007/s00394-019-02039-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02039-9