Abstract
Purpose
Increasing evidence suggests the potential use of natural antioxidant compounds in the prevention/treatment of osteoporosis. This study was undertaken to investigate the effects of purified delphinidin-3-rutinoside (D3R), isolated from Solanum melongena L., on osteoblast viability and differentiation in basal conditions and its ability to protect MC3T3-E1 cells against oxidative damage induced by tert-butyl hydroperoxide (t-BHP).
Methods
MC3T3-E1 osteoblastic cells were treated with D3R (10−11–10−5 M for 24 h), followed by treatment with t-BHP (250 µM for 3 h). To test cell viability, MTT test was performed. Apoptotic cells were stained with Hoechst-33258 dye. Cytoskeleton rearrangement was stained with FICT-labelled phalloidin. Intracellular ROS production was measured using dichlorofluorescein CM-DCFA. The reduced glutathione to oxidized glutathione ratio (GSH/GSSG) contents was measured according to the OPT fluorimetric assay.
Results
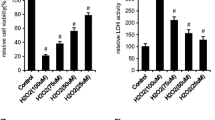
D3R (10−9 M) significantly increases viability of MC3T3-E1 cells and promotes osteoblast differentiation by increasing the expression of type I collagen, alkaline phosphatase and osteocalcin. Pre-treatment with D3R (10−9 M) significantly prevented t-BHP-induced osteoblastic dysfunction and changes in the cytoskeleton organization by decreasing intracellular ROS and preventing the reduction in GSH/GSSG. D3R did not significantly modify the expression of Osteoprotegerin/RANKL system activated by t-BHP suggesting a lack of effect of D3R on osteoblast/osteoclast crosstalk. D3R protective effects against t-BHP-induced osteoblastic dysfunction were mediated by the PI3K/Akt pathway since they were completely prevented by LY294002, a PI3K/Akt specific inhibitor.
Conclusions
These findings indicate that D3R protects MC3T3-E1 cells from oxidative damage and suggest the potential utility of dietary D3R supplement to prevent osteoblast dysfunction in age-related osteoporosis.









Similar content being viewed by others
References
Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H (2007) Trends in oxidative aging theories. Free Radic Biol Med 43(4):477–503
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O’Brien CA, Bellido T, Parfit AM, Weinstein RS, Jilka RL, Manolagas SC (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282(37):27285–27297
Basu S, Michaëlsson K, Olofsson H, Johansson S, Melhus H (2001) Association between oxidative stress and bone mineral density. Biochem Biophys Res Commun 288(1):275–279
D’Amelio P, Cristofaro MA, Tamone C, Morra E, Di Bella S, Isaia G, Grimaldi A, Gennero L, Gariboldi A, Ponzetto A, Pescarmona GP, Isaia GC (2008) Role of iron metabolism and oxidative damage in postmenopausal bone loss. Bone. 43(6):1010–1015. https://doi.org/10.1016/j.bone.2008.08.107
Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R, Cherubini A (2003) Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab 88(4):1523–1527
Jilka RL, Almeida M, Ambrogini E, Han L, Roberson PK, Weinstein RS, Manolagas SC (2010) Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell 9(5):851–867. https://doi.org/10.1111/j.1474-9726.2010.00616.x
Slavin JL, Lloyd B (2012) Health benefits of fruits and vegetables. Adv Nutr 3(4):506–516. https://doi.org/10.3945/an.112.002154
Shen CL, von Bergen V, Chyu MC, Jenkins MR, Mo H, Chen CH, Kwun IS (2012) Fruits and dietary phytochemicals in bone protection. Nutr Res 32(12):897–910. https://doi.org/10.1016/j.nutres.2012.09.018
Hubert PA, Lee SG, Lee SK, Chun OK (2014) Dietary polyphenols, berries, and age-related bone loss: a review based on human, animal, and cell studies. Antioxidants (Basel) 3(1):144–158. https://doi.org/10.3390/antiox3010144
Moriwaki S, Suzuki K, Muramatsu M, Nomura A, Inoue F, Into T, Yoshiko Y, Niida S (2014) Delphinidin, one of the major anthocyanidins, prevents bone loss through the inhibition of excessive osteoclastogenesis in osteoporosis model mice. PLoS One 9(5):e97177. https://doi.org/10.1371/journal.pone.0097177
Hardcastle AC, Aucott L, Reid DM, Macdonald HM (2011) Associations between dietary flavonoid intakes and bone health in a Scottish population. J Bone Miner Res 26(5):941–947. https://doi.org/10.1002/jbmr.285
Sacco SM, Horcajada MN, Offord E (2013) Phytonutrients for bone health during ageing. Br J Clin Pharmacol 75(3):697–707. https://doi.org/10.1111/bcp.12033
Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85(3):632–639
Lean JM, Jagger CJ, Kirstein B, Fuller K, Chambers TJ (2005) Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 146(2):728–735
Rodda SJ, McMahon AP (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133(16):3231–3244
Van der Horst A, Burgering BM (2007) Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol 8(6):440–450
Cao GH, Sofic E, Prior RL (1996) Antioxidant capacity of tea and common vegetables. J Agric Food Chem 44(11):3426–3431
Akanitapichat P, Phraibung K, Nuchklang K, Prompitakkul S (2010) Antioxidant and hepatoprotective activities of five eggplant varieties. Food Chem Toxicol 48(10):3017–3021. https://doi.org/10.1016/j.fct.2010.07.045
Kayamori F, Igarashi K (1994) Effects of dietary nasunin on the serum-cholesterol level in rats. Biosci Biotechnol Biochem 58(3):570–571
Mennella G, Lo Scalzo R, Fibiani M, D’Alessandro A, Francese G, Toppino L, Acciarri N, de Almeida AE, Rotino GL (2012) Chemical and bioactive quality traits during fruit ripening in eggplant (S. melongena L.) and allied species. J Agric Food Chem 60(47):11821–11831
Casati L, Pagani F, Braga PC, Lo Scalzo R, Sibilia V (2016) Nasunin, a new player in the field of osteoblast protection against oxidative stress. J Funct Foods 23:474–484
Yi L, Chen CY, Jin X, Zhang T, Zhou Y, Zhang QY, Zhu JD, Mi MT (2012) Differential suppression of intracellular reactive oxygen species-mediated signaling pathway in vascular endothelial cells by several subclasses of flavonoids. Biochimie 94(9):2035–2044. https://doi.org/10.1016/j.biochi.2012
He J, Giusti MM (2010) Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol 1:163 – 87 doi. https://doi.org/10.1146/annurev.food.080708.100754
Jing P, Qian B, Zhao S, Qi X, Ye L, Mónica Giusti M, Wang X (2015) Effect of glycosylation patterns of Chinese eggplant anthocyanins and other derivatives on antioxidant effectiveness in human colon cell lines. Food Chem 172:183–189. https://doi.org/10.1016/j.foodchem.2014.08.100
Azuma K, Ohyama A, Ippoushi K, Ichiyanagi T, Takeuchi A, Saito T, Fukuoka H (2008) Structures and antioxidant activity of anthocyanins in many accessions of eggplant and its related species. J Agric Food Chem 56(21):10154–10159. https://doi.org/10.1021/jf801322m
Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ (1992) Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res 7(6):683–692
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423(6937):337–342
Mrak E, Casati L, Pagani F, Rubinacci A, Zarattini G, Sibilia V (2015) Ghrelin increases beta-catenin level through protein kinase A activation and regulates OPG expression in rat primary osteoblasts. Int J Endocrinol 2015:547473. https://doi.org/10.1155/2015/547473
Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ (2005) Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem 280(17):17497–17506
Noda Y, Kneyuki T, Igarashi K, Mori A, Packerm L (2000) Antioxidant activity of nasunin, an anthocyanin in eggplant peels. Toxicology 148(2–3):119–123
Braga PC, Lo Scalzo R, Dal Sasso M, Lattuada N, Greco V, Fibiani M (2016) Characterization and antioxidant activity of semi-purified extracts and pure delphinidin-glycosides from eggplant peel (Solanum melongena L.). J Funct Foods 20:411–421
Dieci E, Casati L, Pagani F, Celotti F, Sibilia V (2014) Acylated and unacylated ghrelin protect MC3T3-E1 cells against tert-butyl hydroperoxide-induced oxidative injury: pharmacological characterization of ghrelin receptor and possible epigenetic involvement. Amino Acids 46(7):1715–1725
Zhang JK, Yang L, Meng GL, Fan J, Chen JZ, He QZ, Chen S, Fan JZ, Luo ZJ, Liu J (2012) Protective effect of tetrahydroxystilbene glucoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. Eur J Pharmacol 689(1–3):31–37. https://doi.org/10.1016/j.ejphar.2012.05.045
Dong CL, Liu HZ, Zhang ZC, Zhao HL, Zhao H, Huang Y, Yao JH, Sun TS (2015) The influence of microRNA-150 in osteoblast matrix mineralization. J Cell Biochem 116(12):2970–2979. https://doi.org/10.1002/jcb.25245
Brambilla L, Cantoni O (1998) Mitochondrial formation of hydrogen peroxide is causally linked to the antimycin A-mediated prevention of tert-butylhydroperoxide-induced U937 cell death. FEBS Lett 431(2):245–249
Aon MA, Cortassa S, Marbán E, O’Rourke B (2003) Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278(45):44735–44744. https://doi.org/10.1074/jbc.M302673200
Titorencu I, Pruna V, Jinga VV, Simionescu M (2014) Osteoblast ontogeny and implications for bone pathology: an overview. Cell Tissue Res 355(1):23–33. https://doi.org/10.1007/s00441-013-1750-3
Stein GS, Lian JB, Owen TA (1990) Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J 4(13):3111–3123
Farley JR, Hall SL, Tanner MA, Wergedal JE (1994) Specific activity of skeletal alkaline phosphatase in human osteoblast-line cells regulated by phosphate, phosphate esters, and phosphate analogs and release of alkaline phosphatase activity inversely regulated by calcium. J Bone Miner Res 9(4):497–508
Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382(6590):448–452
Rodan GA, Noda M (1991) Gene expression in osteoblastic cells. Crit Rev Eukaryot Gene Expr 1(2):85–98
Bodine PV, Komm BS (2006) Wnt signaling and osteoblastogenesis. Rev Endocr Metab Disord 7:33–39
Novak A, Dedhar S (1999) Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci 56(5–6):523–537
Westendorf JJ, Kahler RA, Schroeder TM (2004) Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39
Tian Y, Xu Y, Fu Q, He M (2011) Parathyroid hormone regulates osteoblast differentiation in a Wnt/β-catenin-dependent manner. Mol Cell Biochem 355(1–2):211–216. https://doi.org/10.1007/s11010-011-0856-8
Mei G, Zou Z, Fu S, Xia L, Zhou J, Zhang Y, Tuo Y, Wang Z, Jin D (2014) Substance P activates the Wnt signal transduction pathway and enhances the differentiation of mouse preosteoblastic MC3T3-E1 cells. Int J Mol Sci 15(4):6224–6240. https://doi.org/10.3390/ijms15046224
Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, Biswal S (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46(4):443–453
McGonnell IM, Grigoriadis AE, Lam EW, Price JS, Sunters A (2012) A specific role for phosphoinositide 3-kinase and AKT in osteoblasts? Front Endocrinol 3:88. https://doi.org/10.3389/fendo.2012.00088
Wang B, Shravah J, Luo H, Raedschelders K, Chen DD, Ansley DM (2009) Propofol protects against hydrogen peroxide-induced injury in cardiac H9c2 cells via Akt activation and Bcl-2 up-regulation. Biochem Biophys Res Commun 389(1):105–111. https://doi.org/10.1016/j.bbrc.2009.08.097
Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE (2000) Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem 275(15):10761–10766
Smith E, Frenkel B (2005) Glucocorticoids inhibit the transcriptional activity of LEF/TCF in differentiating osteoblasts in a glycogen synthase kinase-3beta-dependent and -independent manner. J Biol Chem 280(3):2388–2394
Sunters A, Armstrong VJ, Zaman G, Kypta RM, Kawano Y, Lanyon LE, Price JS (2010) Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to Ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. J Biol Chem 285(12):8743–8758. https://doi.org/10.1074/jbc.M109.027086
Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z (2007) Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 282(15):11221–11229
Fatokun AA, Stone TW, Smith RA (2008) Responses of differentiated MC3T3-E1 osteoblast-like cells to reactive oxygen species. Eur J Pharmacol 587(1–3):35–41. https://doi.org/10.1016/j.ejphar.2008.03.024
Yi L, Chen CY, Jin X, Mi MT, Yu B, Chang H, Ling WH, Zhang T (2010) Structural requirements of anthocyanins in relation to inhibition of endothelial injury induced by oxidized low-density lipoprotein and correlation with radical scavenging activity. FEBS Lett 584(3):583–90. https://doi.org/10.1016/j.febslet.2009.12.006.
Yoshiki Y, Okubo K, Igarashi K (1995) Chemiluminescence of anthocyanins in the presence of acetaldehyde and tert-butyl hydroperoxide. J Biolumin Chemilumin 10(6):335–338. https://doi.org/10.1002/bio.1170100605
An J, Yang H, Zhang Q, Liu C, Zhao J, Zhang L, Chen B (2016) Natural products for treatment of osteoporosis: the effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci 147:46–58. https://doi.org/10.1016/j.lfs.2016.01.024
An J, Hao D, Zhang Q, Chen B, Zhang R, Wang Y, Yang H (2016) Natural products for treatment of bone erosive diseases: the effects and mechanisms on inhibiting osteoclastogenesis and bone resorption. Int Immunopharmacol 36:118–131. https://doi.org/10.1016/j.intimp.2016.04.024
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med 49(11):1603–1616. https://doi.org/10.1016/j.freeradbiomed.2010.09.006
Knowles HJ, Athanasou NA (2009) Canonical and non-canonical pathways of osteoclast formation. Histol Histopathol 24(3):337–346. https://doi.org/10.14670/HH-24.337
Liu H, Mao P, Wang J, Wang T, Xie CH2 (2016) Azilsartan, an angiotensin II type 1 receptor blocker, attenuates tert-butyl hydroperoxide-induced endothelial cell injury through inhibition of mitochondrial dysfunction and anti-inflammatory activity. Neurochem Int 94:48–56. https://doi.org/10.1016/j.neuint.2016.02.005
Lee SG, Kim B, Yang Y, Pham TX, Park YK, Manatou J, Koo SI, Chun OK, Lee JY (2014) Berry anthocyanins suppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J Nutr Biochem 25(4):404–411. https://doi.org/10.1016/j.jnutbio.2013.12.001
Bakker J, Timberlake CF (1985) The distribution of anthocyanins in grape skin extracts of port wine cultivars as determined by high performance liquid chromatography. J Sci Food Agric 36(12):1315–1324
Acknowledgements
This work was supported by funds from PROGETTO CARIPLO GIOVANI 2015−0834 to Lavinia Casati. The authors thank the expertise and technical support of Dr. Giuseppe L. Rotino (CREA-ORL, Montanaso Lombardo) for providing the aubergine fruits and Prof. Giovanna Speranza (Dipartimento di Chimica, Università degli Studi di Milano) for the analysis by 1H-NMR of D3R crystals purity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Casati, L., Pagani, F., Fibiani, M. et al. Potential of delphinidin-3-rutinoside extracted from Solanum melongena L. as promoter of osteoblastic MC3T3-E1 function and antagonist of oxidative damage. Eur J Nutr 58, 1019–1032 (2019). https://doi.org/10.1007/s00394-018-1618-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1618-0