Abstract
Purpose
Chlorella vulgaris (CV) has exhibited immune-enhancing and protective activities against cancer and infections. However, there is an increasing concern about the use of Chlorella species in human, regarding its various molecules with antigenic features found in infectious microorganisms. Our goal was to investigate the impact of higher concentrations of CV on tumor growth in spontaneous mouse mammary tumor (SMMT) models.
Methods
Balb/c mice were daily given CV powder at doses of 0, 200, or 300 mg/kg for 42 days (CONTROL, CV200, and CV300 groups, respectively; n = 6/group). On day 14, the SMMT was inoculated. Tumor volume (TV) and body weight (BW) were monitored on 5-day intervals following tumor challenge. On day 43, blood, spleen, lungs, and tumor tissues were collected. Histopathological examinations on lungs and tumor tissues were performed following hematoxylin–eosin staining. Intratumor expression of 27 genes was assessed by real-time PCR. Total IgG, IFNγ, and IL-4 levels in serum and spleen culture supernatant were measured by ELISA.
Results
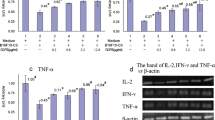
The TV/BW index showed significant increase in the CV200 group compared to the CONTROL (p = 0.047). The CV200 tumors exhibited more malignant phenotype, higher angiogenesis rate, and lower peritumoral neutrophil and macrophage-to-lymphocyte infiltration ratio compared to the CONTROL. Serum concentrations of IFNγ, IL-4, and IgG were declined, and the spleen IFNγ and IgG production was higher in the CV200 compared to the CONTROL. The IL-1β, IL-10, TGFβ1, FOXP3, HO-1, Gr1, CD11b, PCNA, LCN2, iNOS2, VEGFR2, CD31, and CD105L expressions were markedly increased in the CV200 tumors compared to the CONTROL (p = 0.001, 0.002, 0.006, 0.021, 0.004, 0.030, 0.016, 0.031, 0.025, 0.008, 0.014, 0.022, and 0.037, respectively). The changes in cytokine, IgG and gene expression values considerably correlated with tumor size, as well as with each other.
Conclusions
Our data provided evidence that C. vulgaris at a specific dose (200 mg/kg) promoted tumor growth in a mammary tumor model. This consequence might reflect an immune derangement in favor of developing a protumor microenvironment. However, this hypothesis needs to be further investigated in future.








Similar content being viewed by others
References
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. doi:10.1016/j.cell.2011.02.013
Goldszmid RS, Dzutsev A, Trinchieri G (2014) Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe 15(3):295–305. doi:10.1016/j.chom.2014.02.003
Voisin MB, Buzoni-Gatel D, Bout D, Velge-Roussel F (2004) Both expansion of regulatory GR1 + CD11b + myeloid cells and anergy of T lymphocytes participate in hyporesponsiveness of the lung-associated immune system during acute toxoplasmosis. Infect Immun 72(9):5487–5492. doi:10.1128/IAI.72.9.5487-5492.2004
Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, Voronov E, Apte RN (2005) CD11b+/Gr-1 + immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol 175(12):8200–8208. doi:10.4049/jimmunol.175.12.8200
Wang H-M, Pan J-L, Chen C-Y, Chiu C-C, Yang M-H, Chang H-W, Chang J-S (2010) Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem 45(12):1865–1872. doi:10.1016/j.procbio.2010.05.023
Queiroz Jde S, Barbosa CM, Rocha MC, Bincoletto C, Paredes-Gamero EJ, Queiroz ML, Palermo Neto J (2013) Chlorella vulgaris treatment ameliorates the suppressive effects of single and repeated stressors on hematopoiesis. Brain Behav Immun 29:39–50. doi:10.1016/j.bbi.2012.12.001
Kang H, Lee CH, Kim JR, Kwon JY, Seo SG, Han JG, Kim BG, Kim JE, Lee KW (2015) Chlorella vulgaris attenuates dermatophagoides farinae-induced atopic dermatitis-like symptoms in NC/Nga mice. Int J Mol Sci 16(9):21021–21034. doi:10.3390/ijms160921021
Vecina JF, Oliveira AG, Araujo TG, Baggio SR, Torello CO, Saad MJ, Queiroz ML (2014) Chlorella modulates insulin signaling pathway and prevents high-fat diet-induced insulin resistance in mice. Life Sci 95(1):45–52. doi:10.1016/j.lfs.2013.11.020
Justo GZ, Silva MR, Queiroz ML (2001) Effects of the green algae Chlorella vulgaris on the response of the host hematopoietic system to intraperitoneal ehrlich ascites tumor transplantation in mice. Immunopharmacol Immunotoxicol 23(1):119–132. doi:10.1081/IPH-100102573
Ramos AL, Torello CO, Queiroz ML (2010) Chlorella vulgaris modulates immunomyelopoietic activity and enhances the resistance of tumor-bearing mice. Nutr Cancer 62(8):1170–1180. doi:10.1080/01635581.2010.513801
Souza Queiroz J, Marin Blasco I, Gagliano H, Daviu N, Gomez Roman A, Belda X, Carrasco J, Rocha MC, Palermo Neto J, Armario A (2016) Chlorella vulgaris reduces the impact of stress on hypothalamic-pituitary-adrenal axis and brain c-fos expression. Psychoneuroendocrinology 65:1–8. doi:10.1016/j.psyneuen.2015.12.002
Kwak JH, Baek SH, Woo Y, Han JK, Kim BG, Kim OY, Lee JH (2012) Beneficial immunostimulatory effect of short-term Chlorella supplementation: enhancement of natural killer cell activity and early inflammatory response (randomized, double-blinded, placebo-controlled trial). Nutr J 11:53. doi:10.1186/1475-2891-11-53
Azocar J, Diaz A (2013) Efficacy and safety of Chlorella supplementation in adults with chronic hepatitis C virus infection. World J Gastroenterol 19(7):1085–1090. doi:10.3748/wjg.v19.i7.1085
Noguchi N, Maruyama I, Yamada A (2014) The influence of chlorella and its hot water extract supplementation on quality of life in patients with breast cancer. Evid Based Complement Alternat Med 2014:704619. doi:10.1155/2014/704619
Armstrong PB, Armstrong MT, Pardy RL, Child A, Wainwright N (2002) Immunohistochemical demonstration of a lipopolysaccharide in the cell wall of a eukaryote, the green alga, Chlorella. Biol Bull 203(2):203–204
Suarez ER, Kralovec JA, Grindley TB (2010) Isolation of phosphorylated polysaccharides from algae: the immunostimulatory principle of Chlorella pyrenoidosa. Carbohydr Res 345(9):1190–1204. doi:10.1016/j.carres.2010.04.004
Reyes Suarez E, Bugden SM, Kai FB, Kralovec JA, Noseda MD, Barrow CJ, Grindley TB (2008) First isolation and structural determination of cyclic beta-(1–>2)-glucans from an alga, Chlorella pyrenoidosa. Carbohydr Res 343(15):2623–2633. doi:10.1016/j.carres.2008.07.009
Tabarsa M, Shin I-S, Lee JH, Surayot U, Park W, You S (2015) An immune-enhancing water-soluble α-glucan from Chlorella vulgaris and structural characteristics. Food Sci Biotechnol 24(6):1933–1941. doi:10.1007/s10068-015-0255-0
Hasegawa T, Matsuguchi T, Noda K, Tanaka K, Kumamoto S, Shoyama Y, Yoshikai Y (2002) Toll-like receptor 2 is at least partly involved in the antitumor activity of glycoprotein from Chlorella vulgaris. Int Immunopharmacol 2(4):579–589 doi:10.1016/S1567-5769(02)00002-4
Hsu HY, Jeyashoke N, Yeh CH, Song YJ, Hua KF, Chao LK (2010) Immunostimulatory bioactivity of algal polysaccharides from Chlorella pyrenoidosa activates macrophages via Toll-like receptor 4. J Agric Food Chem 58(2):927–936. doi:10.1021/jf902952z
Pabst O, Mowat AM (2012) Oral tolerance to food protein. Mucosal Immunol 5(3):232–239. doi:10.1038/mi.2012.4
Eroukhmanoff L, Oderup C, Ivars F (2009) T-cell tolerance induced by repeated antigen stimulation: selective loss of Foxp3- conventional CD4 T cells and induction of CD4 T-cell anergy. Eur J Immunol 39(4):1078–1087. doi:10.1002/eji.200838653
Noori S, Taghikhani M, Hassan ZM, Allameha A, Mostafaei A (2010) Tehranolide molecule modulates the immune response, reduce regulatory T cell and inhibits tumor growth in vivo. Mol Immunol 47(7–8):1579–1584. doi:10.1016/j.molimm.2010.01.007
Kruisbeek AM (2001) Isolation of Mouse Mononuclear Cells. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W (eds) Current protocols in immunology. Wiley, New York. doi:10.1002/0471142735.im0301s39
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Anderson P, Carrillo-Galvez AB, Garcia-Perez A, Cobo M, Martin F (2013) CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS One 8(10):e76979. doi:10.1371/journal.pone.0076979
Kim S-H, Hong J-H, Lee Y-C (2013) Ursolic acid, a potential PPARγ agonist, suppresses ovalbumin-induced airway inflammation and Penh by down-regulating IL-5, IL-13, and IL-17 in a mouse model of allergic asthma. Eur J Pharmacol 701(1–3):131–143. doi:10.1016/j.ejphar.2012.11.033
Xiong W, Frasch SC, Thomas SM, Bratton DL, Henson PM (2013) Induction of TGF-beta1 synthesis by macrophages in response to apoptotic cells requires activation of the scavenger receptor CD36. PLoS One 8(8):e72772. doi:10.1371/journal.pone.0072772
Chen Y, Huang F, Wang D, Weng Z, Deng Z (2013) Upregulation of heme oxygenase-1 expression may facilitate memory and learning in mice. Exp Ther Med 5(5):1491–1495. doi:10.3892/etm.2013.995
Patil K, Bellner L, Cullaro G, Gotlinger KH, Dunn MW, Schwartzman ML (2008) Heme oxygenase-1 induction attenuates corneal inflammation and accelerates wound healing after epithelial injury. Invest Ophthalmol Vis Sci 49(8):3379–3386. doi:10.1167/iovs.07-1515
Perez-Gomez E, Eleno N, Lopez-Novoa JM, Ramirez JR, Velasco B, Letarte M, Bernabeu C, Quintanilla M (2005) Characterization of murine S-endoglin isoform and its effects on tumor development. Oncogene 24(27):4450–4461 doi:10.1038/sj.onc.1208644
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Kubatka P, Kapinova A, Kruzliak P, Kello M, Vybohova D, Kajo K, Novak M, Chripkova M, Adamkov M, Pec M, Mojzis J, Bojkova B, Kassayova M, Stollarova N, Dobrota D (2015) Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition 31(4):560–569. doi:10.1016/j.nut.2014.08.010
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125(Pt 23):5591–5596. doi:10.1242/jcs.116392
Fridman WH, Pages F, Sautes-Fridman C, Galon J (2012) The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12(4):298–306. doi:10.1038/nrc3245
Du JW, Xu KY, Fang LY, Qi XL (2012) Interleukin-17, produced by lymphocytes, promotes tumor growth and angiogenesis in a mouse model of breast cancer. Mol Med Rep 6(5):1099–1102. doi:10.3892/mmr.2012.1036
Hagerling C, Casbon AJ, Werb Z (2015) Balancing the innate immune system in tumor development. Trends Cell Biol 25(4):214–220. doi:10.1016/j.tcb.2014.11.001
Esquivel-Velazquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J (2015) The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res 35(1):1–16. doi:10.1089/jir.2014.0026
Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC (2008) Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14(5):408–419. doi:10.1016/j.ccr.2008.10.011
Was H, Dulak J, Jozkowicz A (2010) Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets 11(12):1551–1570. doi:10.2174/1389450111009011551
Mittal K, Ebos J, Rini B (2014) Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol 41(2):235–251. doi:10.1053/j.seminoncol.2014.02.007
Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 182(8):4499–4506. doi:10.4049/jimmunol.0802740
Sapino A, Bongiovanni M, Cassoni P, Righi L, Arisio R, Deaglio S, Malavasi F (2001) Expression of CD31 by cells of extensive ductal in situ and invasive carcinomas of the breast. J Pathol 194(2):254–261. doi:10.1002/1096-9896(200106)194:2<254::AID-PATH880>3.0.CO;2-2
Paauwe M, Heijkants RC, Oudt CH, van Pelt GW, Cui C, Theuer CP, Hardwick JC, Sier CF, Hawinkels LJ (2016) Endoglin targeting inhibits tumor angiogenesis and metastatic spread in breast cancer. Oncogene 35(31):4069–4079. doi:10.1038/onc.2015.509
Turner N, Grose R (2010) Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10(2):116–129
Kohrmann A, Kammerer U, Kapp M, Dietl J, Anacker J (2009) Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer 9:188. doi:10.1186/1471-2407-9-188
Fernandez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA (2005) The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res 11(15):5390–5395. doi:10.1158/1078-0432.CCR-04-2391
Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, Strong RK, Zurakowski D, Moses MA (2009) Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci USA 106(10):3913–3918. doi:10.1073/pnas.0810617106
Oren B, Urosevic J, Mertens C, Mora J, Guiu M, Gomis RR, Weigert A, Schmid T, Grein S, Brune B, Jung M (2016) Tumour stroma-derived lipocalin-2 promotes breast cancer metastasis. J Pathol 239(3):274–285. doi:10.1002/path.4724
Dillehay KL, Lu S, Dong Z (2014) Anti-tumor effects of a novel small molecule targeting PCNA chromatin association in prostate cancer. Mol Cancer Ther 13(12):2817–2826 doi:molcanther.0522.2014
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570. doi:10.1126/science.1203486
Zaidi MR, Merlino G (2011) The two faces of interferon-gamma in cancer. Clin Cancer Res 17(19):6118–6124. doi:10.1158/1078-0432.CCR-11-0482
Hemon P, Jean-Louis F, Ramgolam K, Brignone C, Viguier M, Bachelez H, Triebel F, Charron D, Aoudjit F, Al-Daccak R, Michel L (2011) MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J Immunol 186(9):5173–5183. doi:10.4049/jimmunol.1002050
Bernabei P, Coccia EM, Rigamonti L, Bosticardo M, Forni G, Pestka S, Krause CD, Battistini A, Novelli F (2001) Interferon-gamma receptor 2 expression as the deciding factor in human T, B, and myeloid cell proliferation or death. J Leukoc Biol 70(6):950–960
Li Z, Chen L, Qin Z (2009) Paradoxical roles of IL-4 in tumor immunity. Cell Mol Immunol 6(6):415–422. doi:10.1038/cmi.2009.53
Queiroz ML, da Rocha MC, Torello CO, de Souza Queiroz J, Bincoletto C, Morgano MA, Romano MR, Paredes-Gamero EJ, Barbosa CM, Calgarotto AK (2011) Chlorella vulgaris restores bone marrow cellularity and cytokine production in lead-exposed mice. Food Chem Toxicol 49(11):2934–2941. doi:10.1016/j.fct.2011.06.056
Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S (2009) Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol 85(6):996–1004. doi:10.1189/jlb.0708446
Khalilnezhad A, Mahmoudian E, Mosaffa N, Mohsenifar J, Amani D (2016) Spontaneous mouse mammary tumor cell lysates induce IgG production in spleen mononuclear cells of healthy and tumor-bearing mice. J Immunoassay Immunochem. doi:10.1080/15321819.2016.1266499
MacSween JM, Eastwood SL (1980) Immunoglobulins associated with human tumours in vivo: IgG concentrations in eluates of colonic carcinomas. Br J Cancer 42(4):503–509
Huber SA, Bigi G, Lucas ZJ (1980) Tumor-specific suppressor cells induced by immunization with spleen cells from tumor-bearing animals. Cancer Res 40(10):3477–3483
Toge T, Kameda A, Yamada H, Seto Y, Aratani K, Fujita T, Hattori T (1986) Role of the spleen on immunosuppression in esophageal and gastric cancer. The Japanese journal of surgery 16(5):330–335. doi:10.1007/bf02470555
Nyhus JK, Wolford CC, Friece CR, Nelson MB, Sampsel JW, Barbera-Guillem E (2001) IgG-recognizing shed tumor-associated antigens can promote tumor invasion and metastasis. Cancer Immunol Immunother 50(7):361–372
Barbera-Guillem E, Nyhus JK, Wolford CC, Friece CR, Sampsel JW (2002) Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res 62(23):7042–7049
Dong B, Dai G, Xu L, Zhang Y, Ling L, Sun L, Lv J (2014) Tumor cell lysate induces the immunosuppression and apoptosis of mouse immunocytes. Mol Med Rep 10 (6):2827–2834. doi:10.3892/mmr.2014.2606
Acknowledgements
This study was financially supported by a Grant (No. 1802) from Shahid Beheshti University of Medical Sciences. The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khalilnezhad, A., Mahmoudian, E., Mosaffa, N. et al. Effects of Chlorella vulgaris on tumor growth in mammary tumor-bearing Balb/c mice: discussing association of an immune-suppressed protumor microenvironment with serum IFNγ and IgG decrease and spleen IgG potentiation. Eur J Nutr 57, 1025–1044 (2018). https://doi.org/10.1007/s00394-017-1387-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-017-1387-1