Abstract
Purpose
The objective of the present study was to test the hypothesis that N-acetylcysteine (NAC) may play beneficial roles against intrauterine growth retardation (IUGR)-induced hepatic damage in suckling piglets.
Methods
Fourteen IUGR and seven normal birth weight (NBW) neonatal male piglets were selected. Piglets were weaned at 7 days of postnatal age and fed the control formula milk (NBW-CON and IUGR-CON groups) or the control formula milk supplemented with 1.2 g/kg NAC (IUGR-NAC group) for 14 days (n = 7). The plasma and liver samples were analyzed for the parameters related to hepatic damage, redox status, apoptosis, and autophagy.
Results
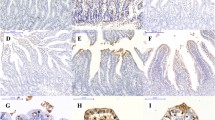
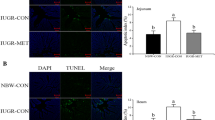
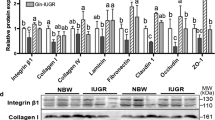
Compared with the NBW-CON group, IUGR-CON group exhibited increased activities of plasma aminotransferases, increased numbers of apoptotic hepatocytes, as well as higher concentrations of protein carbonyl, malondialdehyde (MDA), microtubule-associated protein 1 light chain 3 beta, and phospholipid-conjugated form (MAP1LC3B-II), along with a decrease in the content of reduced glutathione (GSH). NAC treatment increased GSH content and GSH-to-oxidized GSH ratio in the liver of IUGR-NAC group, most likely owing to the improved activities of γ-glutamine-cysteine ligase, γ-glutamine-cysteine synthetase, and glutathione reductase. The hepatic protein carbonyl and MDA contents were decreased in the IUGR-NAC group compared with the IUGR-CON group. In addition, NAC-treated piglets had an increased content of B cell lymphoma/leukemia 2 protein, whereas a decreased expression level of MAP1LC3B-II in the liver.
Conclusions
NAC may have beneficial effects in improving GSH synthesis and cellular homeostasis in the liver of IUGR suckling piglets.





Similar content being viewed by others
References
Kelly FJ (1993) Free radical disorders of preterm infants. Br Med Bull 49:668–678
Muller DP (1987) Free radical problems of the newborn. Proc Nutr Soc 46:69–75
Friel JK, Friesen RW, Harding SV et al (2004) Evidence of oxidative stress in full-term healthy infants. Pediatr Res 56:878–882
Ahola T, Fellman V, Kjellmer I (2004) Plasma 8-isoprostane is increased in preterm infants who develop bronchopulmonary dysplasia or periventricular leukomalacia. Pediatr Res 56:88–93
Weinberger B, Watorek K, Strauss R et al (2002) Association of lipid peroxidation with hepatocellular injury in preterm infants. Crit Care 6:521–525
Takagi Y, Nikaido T, Toki T et al (2004) Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch 444:49–55
Biri A, Bozkurt N, Turp A et al (2007) Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Invest 64:187–192
Küster A, Tea I, Ferchaud-Roucher V et al (2011) Cord blood glutathione depletion in preterm infants: correlation with maternal cysteine depletion. PLoS One 6:e27626
Smith CV, Hansen JN, Martin NE et al (1993) Oxidant stress responses in premature infants during exposure to hyperoxia. Pediatr Res 34:360–365
Sullivan JL, Newton RB (1988) Serum antioxidant activity in neonates. Arch Dis Child l63:748–757
Nakano H, Boudjema K, Alexandre E et al (1995) Protective effects of N-acetylcysteine on hypothermic ischemia-reperfusion injury of rat liver. Hepatology 22:539–545
Jahromi MG, Nabavizadeh F, Vahedian J et al (2010) Protective effect of ghrelin on acetaminophen-induced liver injury in rat. Peptides 11:2114–2117
Hashimoto K, Pinkas G, Evans L et al (2012) Protective effect of N-acetylcysteine on liver damage during chronic intrauterine hypoxia in fetal guinea pig. Reprod Sci 19:1001–1009
Morsy MA, Abdalla AM, Mahmoud AM et al (2012) Protective effects of curcumin, α-lipoic acid, and N-acetylcysteine against carbon tetrachloride-induced liver fibrosis in rats. J Physiol Biochem 68:29–35
Park JE, Yang JH, Yoon SJ et al (2002) Lipid peroxidation-mediated cytotoxicity and DNA damage in U937 cells. Biochimie 84:1199–1205
Reliene R, Fischer E, Schiestl RH (2004) Effect of N-acetylcysteine on oxidative DNA damage and the frequency of DNA deletions in atm-deficient mice. Cancer Res 64:5148–5153
Shivalingappa PC, Jin H, Anantharam V et al (2012) N-acetyl cysteine protects against methamphetamine-induced dopaminergic neurodegeneration via modulation of redox status and autophagy in dopaminergic cells. Parkinsons Dis 1:16–21
D’Inca R, Kloareg M, Gras-Le GC et al (2010) Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs. J Nutr 140:925–931
Zhang H, Chen Y, Li Y et al (2014) Medium-chain TAG attenuate hepatic oxidative damage in intra-uterine growth-retarded weanling piglets by improving the metabolic efficiency of the glutathione redox cycle. Br J Nutr 112:876–885
Caperna TJ, Shannon AE, Blomberg LA et al (2010) Identification of protein carbonyls in serum of the fetal and neonatal pig. Comp Biochem Physiol B Biochem Mol Biol 156:189–196
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Placer ZA, Cushman LL, Johnson BC (1996) Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem 16:359–364
Du ZX, Zhang HY, Meng X et al (2009) Role of oxidative stress and intracellular glutathione in the sensitivity to apoptosis induced by proteasome inhibitor in thyroid cancer cells. BMC Cancer 9:56
Zhou Y, Zhang S, Liu C et al (2009) The protection of selenium on ROS mediated-apoptosis by mitochondria dysfunction in cadmium-induced LLC-PK 1 cells. Toxicol In Vitro 23:288–294
Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 74:214–226
Xu J, Zhu X, Wu L et al (2012) MicroRNA-122 suppresses cell proliferation and induces cell apoptosis in hepatocellular carcinoma by directly targeting Wnt/beta-catenin pathway. Liver Int 32:752–760
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Chen Y, Li D, Dai Z et al (2014) l-Methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids 46:1131–1142
Boehm G, Müller DM, Teichmann B et al (1990) Influence of intrauterine growth retardation on parameters of liver function in low birth weight infants. Eur J Pediatr 149:396–398
Ikeda T, Yanaga K, Kishikawa K et al (1992) Ischemic injury in liver transplantation: difference in injury sites between warm and cold ischemia in rats. Hepatology 16:454–461
Burke C, Sinclair K, Cowin G et al (2006) Intrauterine growth restriction due to uteroplacental vascular insufficiency leads to increased hypoxia-induced cerebral apoptosis in newborn piglets. Brain Res 1098:19–25
Baserga M, Bertolotto C, Maclennan NK et al (2004) Uteroplacental insufficiency decreases small intestine growth and alters apoptotic homeostasis in term intrauterine growth retarded rats. Early Hum Dev 79:93–105
Pham TD, MacLennan NK, Chiu CT et al (2003) Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physiol 285:R962–R970
Espino J, Bejarano I, Paredes SD et al (2011) Protective effect of melatonin against human leukocyte apoptosis induced by intracellular calcium overload: relation with its antioxidant actions. J Pineal Res 51:195–206
Butterfield DA, Stadtman ER (1997) Protein oxidation processes in aging brain. Adv Cell Aging Gerontol 2:161–191
Rajendra-Prasad N, Menon VP, Vasudev V et al (2005) Radioprotective effect of sesamol on g-radiation induced DNA damage, lipid peroxidation and antioxidants levels in cultured human lymphocytes. Toxicology 209:225–235
Dalton TP, Chen Y, Schneider SN et al (2004) Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med 37:1511–1526
Marengo B, De Ciucis C, Verzola D et al (2008) Mechanisms of BSO (l-buthionine-S, R-sulfoximine)-induced cytotoxic effects in neuroblastoma. Free Radic Biol Med 44:474–482
Lee YS, Chou YH (2005) Antioxidant profiles in full term and preterm neonates. Chang Gung Med J 28:846–851
Raab EL, Vuguin PM, Stoffers DA et al (2009) Neonatal exendin-4 treatment reduces oxidative stress and prevents hepatic insulin resistance in intrauterine growth-retarded rats. Am J Physiol Regul Integr Comp Physiol 297:R1785–R1794
Jay FH, Dickinson DA (2003) Oxidative signaling and glutathione synthesis. BioFactors 17:1–12
MacKay DS, Brophy JD, McBreairty LE et al (2012) Intrauterine growth restriction leads to changes in sulfur amino acid metabolism, but not global DNA methylation, in Yucatan miniature piglets. J Nutr Biochem 23:1121–1127
MacLennan NK, James SJ, Melnyk S et al (2004) Uteroplacental insufficiency alters DNA methylation, one-carbon metabolism, and histone acetylation in IUGR rats. Physiol Genomics 18:43–50
Armstrong JS, Whiteman M, Yang H et al (2004) Cysteine starvation activates the redox-dependent mitochondrial permeability transition in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 45:4183–4189
Te Braake FWJ, Schierbeek H, Vermes A et al (2009) High-dose cysteine administration does not increase synthesis of the antioxidant glutathione preterm infants. Pediatrics 124:e978–e984
Anderson ME, Meister A (1987) Intracellular delivery of cysteine. Methods Enzymol 143:313–325
Anderson ME, Luo JL (1998) Glutathione therapy: from prodrugs to genes. Semin Liver Dis 18:415–423
Bernard GR, Lucht WD, Niedermeyer ME et al (1984) Effect of N-acetylcysteine on the pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J Clin Invest 73:1772–1784
Brunet J, Boily MJ, Cordeau S et al (1995) Effects of N-acetylcysteine in the rat heart reperfused after low-flow ischemia: evidence for a direct scavenging of hydroxyl radicals and a nitric oxide-dependent increase in coronary flow. Free Radic Biol Med 19:627–638
Kaeys R, Harrison PM, Wendon JA et al (1991) Intravenous acetylcysteine in paracetamol induced fulminant hepatic failure: a prospective controlled trial. BMJ 303:1026–1029
Mansour HH, Kiki SME, Hasan HF (2015) Protective effect of N-acetylcysteine on cyclophosphamide-induced cardiotoxicity in rats. Environ Toxicol Pharmacol 40:417–422
Shen HM, Yang CF, Ding WX et al (2001) Superoxide radical-initiated apoptotic signalling pathway in selenite-treated HepG 2 cells: mitochondria serve as the main target. Free Radic Biol Med 30:9–21
Santra A, Chowdhury A, Ghatak S et al (2007) Arsenic induces apoptosis in mouse liver is mitochondria dependent and is abrogated by N-acetylcysteine. Toxicol Appl Pharmacol 220(2):146–155
Prunell GF, Arboleda VA, Troy CM (2005) Caspase function in neuronal death: delineation of the role of caspases in ischemia. Curr Drug Targets CNS Neurol Disord 4:51–61
Junn E, Mouradian MM (2001) Apoptotic signaling in dopamine-induced cell death: the role of oxidative stress, p38 mitogenactivated protein kinase, cytochrome c and caspases. J Neurochem 78:374–383
Elphick LM, Hawat M, Toms NJ et al (2008) Opposing roles for caspase and calpain death proteases in l-glutamate-induced oxidative neurotoxicity. Toxicol Appl Pharmacol 232:258–267
Wang C, Chen K, Xia Y et al (2014) N-acetylcysteine attenuates ischemia-reperfusion-induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway. PLoS One 9:e108855
Vendemiale G, Grattagliano I, Caruso ML et al (2001) Increased oxidative stress in dimethylnitrosamine-induced liver fibrosis in the rat: effect of N-acetylcysteine and interferon-alpha. Toxicol Appl Pharmacol 175:130–139
Tanida I, Tanida-Miyake E, Ueno T et al (2001) The human homolog of Saccharomyces cerevisiae Apg7p is a protein-activating enzyme for multiple substrates including human Apg12p, GATE-16, GABARAP, and MAP-LC3. J Biol Chem 276:1701e6
Yuan H, Perry CN, Huang C et al (2009) LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol 296:H470–H479
Yang L, Tan P, Zhou W et al (2012) N-acetylcysteine protects against hypoxia mimetic-induced autophagy by targeting the HIF-1α pathway in retinal ganglion cells. Cell Mol Neurobiol 32:1275–1285
Acknowledgments
The research was financially supported by the National Basic Research Program of People’s Republic of China (973) (Grant No. 2012CB124703).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The use of animals for this research was approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., Su, W., Ying, Z. et al. N-acetylcysteine attenuates intrauterine growth retardation-induced hepatic damage in suckling piglets by improving glutathione synthesis and cellular homeostasis. Eur J Nutr 57, 327–338 (2018). https://doi.org/10.1007/s00394-016-1322-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1322-x