Abstract
Purpose
Limited clinical evidence is available on the effects of amount and types of dietary fats on postprandial insulinemic and gastrointestinal peptide responses in metabolic syndrome subjects. We hypothesized that meals enriched with designated: (1) amount of fats (50 vs 20 g), (2) fats with differing fatty acid composition (saturated, SFA; monounsaturated, MUFA or n-6 polyunsaturated fatty acids, PUFA) would affect insulinemic and gastrointestinal peptide releases in metabolic syndrome subjects.
Methods
Using a randomized, crossover and double-blinded design, 15 men and 15 women with metabolic syndrome consumed high-fat meals enriched with SFA, MUFA or n-6 PUFA, or a low-fat/high-sucrose (SUCR) meal. C-peptide, insulin, glucose, gastrointestinal peptides and satiety were measured up to 6 h.
Results
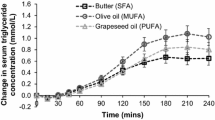
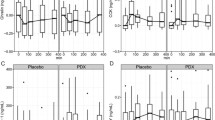
As expected, SUCR meal induced higher C-peptide (45 %), insulin (45 %) and glucose (49 %) responses compared with high-fat meals regardless of types of fatty acids (P < 0.001). Interestingly, incremental area under the curve (AUC0-120min) for glucagon-like peptide-1 was higher after SUCR meal compared with MUFA (27 %) and n-6 PUFA meals (23 %) (P = 0.01). AUC0-120min for glucose-dependent insulinotropic polypeptide was higher after SFA meal compared with MUFA (23 %) and n-6 PUFA meals (20 %) (P = 0.004). Significant meal x time interaction (P = 0.007) was observed for ghrelin, but not cholecystokinin and satiety.
Conclusions
The amount of fat regardless of the types of fatty acids affects insulin and glycemic responses. Both the amount and types of fatty acids acutely affect the gastrointestinal peptide release in metabolic syndrome subjects, but not satiety.



Similar content being viewed by others
Abbreviations
- SFA:
-
Saturated fatty acids
- MUFA:
-
Monounsaturated fatty acids
- PUFA:
-
Polyunsaturated fatty acids
- SUCR:
-
High sucrose/low fat
- GLP-1:
-
Glucagon-like peptide-1
- GIP:
-
Glucose-dependent insulinotropic peptide
- CCK:
-
Cholecystokinin
- AUC:
-
Area under the curve
- VAS:
-
Visual analogue scale
References
Misra A, Singhal N, Khurana L (2010) Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: role of dietary fats and oils. J Am Coll Nutr 29(3 Suppl):289S–301S
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC, International Diabetes Federation Task Force on E, Prevention, Hational Heart L, Blood I, American Heart A, World Heart F, International Atherosclerosis S, International Association for the Study of O (2009) Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120(16):1640–1645
Kallio P, Kolehmainen M, Laaksonen DE, Pulkkinen L, Atalay M, Mykkanen H, Uusitupa M, Poutanen K, Niskanen L (2008) Inflammation markers are modulated by responses to diets differing in postprandial insulin responses in individuals with the metabolic syndrome. Am J Clin Nutr 87(5):1497–1503
Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, Nalsen C, Berglund L, Louheranta A, Rasmussen BM, Calvert GD, Maffetone A, Pedersen E, Gustafsson IB, Storlien LH, Study K (2001) Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU study. Diabetologia 44(3):312–319
Jebb SA, Lovegrove JA, Griffin BA, Frost GS, Moore CS, Chatfield MD, Bluck LJ, Williams CM, Sanders TA, Group RS (2010) Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (reading, imperial, surrey, cambridge, and kings) trial. Am J Clin Nutr 92(4):748–758
Lopez S, Bermudez B, Ortega A, Varela LM, Pacheco YM, Villar J, Abia R, Muriana FJ (2011) Effects of meals rich in either monounsaturated or saturated fat on lipid concentrations and on insulin secretion and action in subjects with high fasting triglyceride concentrations. Am J Clin Nutr 93(3):494–499
Summers LK, Fielding BA, Bradshaw HA, Ilic V, Beysen C, Clark ML, Moore NR, Frayn KN (2002) Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 45(3):369–377
de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, Uleryk E, Budylowski P, Schunemann H, Beyene J, Anand SS (2015) Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 351:h3978
Meloni AR, DeYoung MB, Lowe C, Parkes DG (2013) GLP-1 receptor activated insulin secretion from pancreatic beta-cells: mechanism and glucose dependence. Diabetes Obes Metab 15(1):15–27
Getty-Kaushik L, Song DH, Boylan MO, Corkey BE, Wolfe MM (2006) Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity (Silver Spring) 14(7):1124–1131
Gibbons C, Caudwell P, Finlayson G, Webb DL, Hellstrom PM, Naslund E, Blundell JE (2013) Comparison of Postprandial Profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J Clin Endocrinol Metab 98(5):E847–E855. doi:10.1210/jc.2012-3835
Matzinger D, Degen L, Drewe J, Meuli J, Duebendorfer R, Ruckstuhl N, D’Amato M, Rovati L, Beglinger C (2000) The role of long chain fatty acids in regulating food intake and cholecystokinin release in humans. Gut 46(5):688–693
Hunt JN, Knox MT (1968) A relation between the chain length of fatty acids and the slowing of gastric emptying. J Physiol 194(2):327–336
Lardinois CK, Starich GH, Mazzaferri EL (1988) The postprandial response of gastric inhibitory polypeptide to various dietary fats in man. J Am Coll Nutr 7(3):241–247
Feltrin KL, Little TJ, Meyer JH, Horowitz M, Smout AJ, Wishart J, Pilichiewicz AN, Rades T, Chapman IM, Feinle-Bisset C (2004) Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol 287(3):R524–R533
Bagger JI, Knop FK, Lund A, Vestergaard H, Holst JJ, Vilsboll T (2011) Impaired regulation of the incretin effect in patients with type 2 diabetes. J Clin Endocrinol Metab 96(3):737–745
Vilsboll T, Krarup T, Sonne J, Madsbad S, Volund A, Juul AG, Holst JJ (2003) Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab 88(6):2706–2713
Teng KT, Chang CY, Kanthimathi MS, Tan AT, Nesaretnam K (2015) Effects of amount and type of dietary fats on postprandial lipemia and thrombogenic markers in individuals with metabolic syndrome. Atherosclerosis 242(1):281–287
Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 5:600–608
Parker BA, Sturm K, MacIntosh CG, Feinle C, Horowitz M, Chapman IM (2004) Relation between food intake and visual analogue scale ratings of appetite and other sensations in healthy older and young subjects. Eur J Clin Nutr 58(2):212–218
Min JY, Min KB (2013) Serum C-peptide levels as an independent predictor of diabetes mellitus mortality in non-diabetic individuals. Eur J Epidemiol 28(9):771–774
Xiao C, Giacca A, Carpentier A, Lewis GF (2006) Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia 49(6):1371–1379
Masson CJ, Mensink RP (2011) Exchanging saturated fatty acids for (n-6) polyunsaturated fatty acids in a mixed meal may decrease postprandial lipemia and markers of inflammation and endothelial activity in overweight men. J Nutr 141(5):816–821
Teng KT, Nagapan G, Cheng HM, Nesaretnam K (2011) Palm olein and olive oil cause a higher increase in postprandial lipemia compared with lard but had no effect on plasma glucose, insulin and adipocytokines. Lipids 46(4):381–388
Filippou A, Berry SE, Baumgartner S, Mensink RP, Sanders TA (2014) Palmitic acid in the sn-2 position decreases glucose-dependent insulinotropic polypeptide secretion in healthy adults. Eur J Clin Nutr 68(5):549–554
Lopez S, Bermudez B, Pacheco YM, Villar J, Abia R, Muriana FJ (2008) Distinctive postprandial modulation of beta cell function and insulin sensitivity by dietary fats: monounsaturated compared with saturated fatty acids. Am J Clin Nutr 88(3):638–644
Zang H, Carlstrom K, Arner P, Hirschberg AL (2006) Effects of treatment with testosterone alone or in combination with estrogen on insulin sensitivity in postmenopausal women. Fertil Steril 86(1):136–144
Ek I, Arner P, Ryden M, Holm C, Thorne A, Hoffstedt J, Wahrenberg H (2002) A unique defect in the regulation of visceral fat cell lipolysis in the polycystic ovary syndrome as an early link to insulin resistance. Diabetes 51(2):484–492
Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ, Hansen HS (2011) 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab 96(9):E1409–E1417
Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11(1):90–94
Ichimura A, Hirasawa A, Hara T, Tsujimoto G (2009) Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat 89(3–4):82–88
Lynn FC, Thompson SA, Pospisilik JA, Ehses JA, Hinke SA, Pamir N, McIntosh CH, Pederson RA (2003) A novel pathway for regulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in beta cells. FASEB J 17(1):91–93
Lardinois CK, Starich GH, Mazzaferri EL, DeLett A (1987) Polyunsaturated fatty acids augment insulin secretion. J Am Coll Nutr 6(6):507–515
Lardinois CK, Starich GH, Mazzaferri EL, DeLett A (1988) Effect of source of dietary fats on serum glucose, insulin, and gastric inhibitory polypeptide responses to mixed test meals in subjects with non-insulin dependent diabetes mellitus. J Am Coll Nutr 7(2):129–136
Ross SA, Shaffer EA (1981) The importance of triglyceride hydrolysis for the release of gastric inhibitory polypeptide. Gastroenterology 80(1):108–111
Flatt PR, Bailey CJ, Kwasowski P, Page T, Marks V (1984) Plasma immunoreactive gastric inhibitory polypeptide in obese hyperglycaemic (ob/ob) mice. J Endocrinol 101(3):249–256
Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J, Hermansen K (1999) Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr 69(6):1135–1143
Thomsen C, Storm H, Holst JJ, Hermansen K (2003) Differential effects of saturated and monounsaturated fats on postprandial lipemia and glucagon-like peptide 1 responses in patients with type 2 diabetes. Am J Clin Nutr 77(3):605–611
Al Massadi O, Lear PV, Muller TD, Lopez M, Dieguez C, Tschop MH, Nogueiras R (2014) Review of novel aspects of the regulation of ghrelin secretion. Curr Drug Metab 15(4):398–413
Al Massadi O, Pardo M, Roca-Rivada A, Castelao C, Casanueva FF, Seoane LM (2010) Macronutrients act directly on the stomach to regulate gastric ghrelin release. J Endocrinol Invest 33(9):599–602
Goff LM, Griffin BA, Lovegrove JA, Sanders TA, Jebb SA, Bluck LI, Frost GS, RS Group (2013) Ethnic differences in beta-cell function, dietary intake and expression of the metabolic syndrome among UK adults of South Asian, black African-Caribbean and white-European origin at high risk of metabolic syndrome. Diab Vasc Dis Res 10(4):315–323
Acknowledgments
We thank MPOB and director general of MPOB for the study funding and permission to publish this manuscript. Our gratitude goes to Prof. Gary Frost in reviewing the manuscript. We also thank the staff and students from Nutrition Unit, MPOB for their help in this study. We are grateful to the subjects for their participation. This study was supported by a grant from the Malaysian Palm Oil Board, and by High-Impact Research grant, UM.C/HIR/MOHE/MED/11(H-20001-E000043) from the Ministry of Higher Education and University of Malaya, Malaysia.
Author’s contribution
Teng KT. designed the research protocol; Teng KT. and Chang CY conducted the research including subject recruitment and postprandial challenges, analyzed data and wrote the paper. Teng KT, Chang CY, Kanthimathi MS, Nesaretnam K and Tan ATB contributed to manuscript writing. Teng KT had primary responsibility for final content. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Teng KT and Nesaretnam K are providing consulting services to Malaysian Palm Oil Board (MPOB). Tan ATB, Kanthimathi MS and Chang CY have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, CY., Kanthimathi, M.S., Tan, A.TB. et al. The amount and types of fatty acids acutely affect insulin, glycemic and gastrointestinal peptide responses but not satiety in metabolic syndrome subjects. Eur J Nutr 57, 179–190 (2018). https://doi.org/10.1007/s00394-016-1307-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1307-9