Abstract
Purpose
A deficient total sulfur amino acid (TSAA) supply has been reported to differently affect the amino acid composition of tissues, but limited information is available about its effects on the morphology and metabolic properties of splanchnic tissues.
Methods
The amino acid composition, protein metabolism, glutathione concentration of the liver, proximal and distal jejunum, ileum and kidneys, and intestinal architecture were compared in 42-day-old piglets pair-fed either a diet deficient (TSAA−; 28 % deficiency) or sufficient (TSAA+) in TSAA for 10 days.
Results
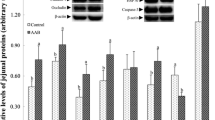
The supply of TSAA had no effect on tissue weights, but influenced the amino acid composition in a tissue-dependent manner. Compared with animals receiving diet TSAA+, the concentrations of Met and Ser were higher in liver protein of TSAA− animals while the Cys concentration in protein was lower in the liver but higher in the distal jejunum. The TSAA supply had no effect on protein synthesis and proteolytic activities of tissues. Villus width and surface, and crypt surface were lower in the proximal jejunum of TSAA− versus TSAA+ pigs. Crypt surface in the ileum of TSAA− pigs was higher. Pigs receiving diet TSAA− had lower GSH and GSSG concentrations in the liver and proximal jejunum, but the GSH/GSSG ratio was decreased only in the liver.
Conclusions
A greater nutritional priority appears to be given to splanchnic tissues so that its growth and protein metabolism can be maintained when the TSAA supply is limiting. The amino acid composition, glutathione status, and intestinal mucosa architecture are affected in a tissue-dependent manner.

Similar content being viewed by others
References
NRC (1998) Nutrient requirement of swine, 10th edn. National Academic Press, Washington
Chung TK, Baker DH (1992) Efficiency of dietary methionine utilization by young pigs. J Nutr 122(9):1862–1869
Kim YI (2005) Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr 135(11):2703–2709
Lushchak VI (2012) Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids 2012:736837. doi:10.1155/2012/736837
Conde-Aguilera JA, Barea R, Le Floc’h N, Lefaucheur L, van Milgen J (2010) A sulfur amino acid deficiency changes the amino acid composition of body protein in piglets. Animal 4(8):1349–1358. doi:10.1017/S1751731110000340
Seve B, Ponter AA (1997) Nutrient-hormone signals regulating muscle protein turnover in pigs. Proc Nutr Soc 56(2):565–580. doi:10.1079/pns19970058
Lobley GE (1993) Species comparisons of tissue protein-metabolim—effects of age and hormonal action. J Nutr 123(2):337–343
Sugden PH, Fuller SJ (1991) Regulation of protein turnover in skeletal and cardiac muscle. Biochem J 273(Pt 1):21–37
Tesseraud S, Peresson R, Lopes J, Chagneau AM (1996) Dietary lysine deficiency greatly affects muscle and liver protein turnover in growing chickens. Br J Nutr 75(6):853–865
Hamard A, Seve B, Le Floc’h N (2009) A moderate threonine deficiency differently affects protein metabolism in tissues of early-weaned piglets. Comp Biochem Physiol A Mol Integr Physiol 152(4):491–497. doi:10.1016/j.cbpa.2008.12.002
Brosnan JT, Brosnan ME (2006) The sulfur-containing amino acids: an overview. J Nutr 136(6 Suppl):1636S–1640S
Stipanuk MH (2004) Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 24:539–577
Shoveller AK, Stoll B, Ball RO, Burrin DG (2005) Nutritional and functional importance of intestinal sulfur amino acid metabolism. J Nutr 135(7):1609–1612
Riedijk MA, Stoll B, Chacko S, Schierbeek H, Sunehag AL, van Goudoever JB, Burrin DG (2007) Methionine transmethylation and transsulfuration in the piglet gastrointestinal tract. Proc Natl Acad Sci USA 104(9):3408–3413. doi:10.1073/pnas.0607965104
Bauchart-Thevret C, Cottrell J, Stoll B, Burrin DG (2011) First-pass splanchnic metabolism of dietary cysteine in weanling pigs. J Anim Sci 89:4093–4099. doi:10.2527/jas.2011-3944
Bauchart-Thevret C, Stoll B, Chacko S, Burrin DG (2009) Sulfur amino acid deficiency upregulates intestinal methionine cycle activity and suppresses epithelial growth in neonatal pigs. Am J Physiol Endocrinol Metab 296(6):E1239–E1250. doi:10.1152/ajpendo.91021.2008
Hamard A, Mazurais D, Boudry G, Le Huerou-Luron I, Seve B, Le Floc’h N (2010) A moderate threonine deficiency affects gene expression profile, paracellular permeability and glucose absorption capacity in the ileum of piglets. J Nutr Biochem 21(10):914–921. doi:10.1016/j.jnutbio.2009.07.004
Ebner S, Schoknecht P, Reeds P, Burrin D (1994) Growth and metabolism of gastrointestinal and skeletal muscle tissues in protein-malnourished neonatal pigs. Am J Physiol 266(6 Pt 2):R1736–R1743
Conde-Aguilera JA, Lefaucheur L, Tesseraud S, Mercier Y, Le Floc’h N, Van Milgen J (2015) Skeletal muscles respond differently when piglets are offered a diet 30% deficient in total sulfur amino acid for 10 days. Eur J Nutr. doi:10.1007/s00394-014-0830-9
Henry Y (1993) Affinement du concept de la protéine idéale pour le porc en croissance. INRA Prod Anim 6:199–212
Seve B, Ballevre O, Ganier P, Noblet J, Prugnaud J, Obled C (1993) Recombinant porcine somatotropin and dietary protein enhance protein synthesis in growing pigs. J Nutr 123(3):529–540
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106(1):207–212. doi:10.1016/0003-2697(80)90139-6
Goodlad RA, Levi S, Lee CY, Mandir N, Hodgson H, Wright NA (1991) Morphometry and cell-proliferation in endoscopic biopsies—evaluation of a technique. Gastroenterology 101(5):1235–1241
SAS (2004) SAS/STAT 9.1 user’s guide. SAS Institute Inc., Cary
Buddington RK, Elnif J, Puchal-Gardiner AA, Sangild PT (2001) Intestinal apical amino acid absorption during development of the pig. Am J Physiol Regul Integr Comp Physiol 280(1):R241–R247
Faure M, Moennoz D, Montigon F, Mettraux C, Breuille D, Ballevre O (2005) Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats. J Nutr 135(3):486–491
Rivera-Ferre MG, Aguilera JF, Nieto R (2006) Differences in whole-body protein turnover between Iberian and Landrace pigs fed adequate or lysine-deficient diets. J Anim Sci 84(12):3346–3355. doi:10.2527/jas.2005-405
Wang X, Qiao SY, Yin YL, Yue LY, Wang ZY, Wu GY (2007) A deficiency or excess of dietary threonine reduces protein synthesis in jejunum and skeletal muscle of young pigs. J Nutr 137(6):1442–1446
Sève B, Reeds PJ, Fuller MF, Cadenhead A, Hay SM (1986) Protein synthesis and retention in some tissues of the young-pig as influenced by dietary-protein intake after early-weaning. Possible connection to the energy metabolism. Reprod Nutr Dev 26(3):849–861. doi:10.1051/rnd:19860509
Ponter AA, Cortamira NO, Seve B, Salter DN, Morgan LM (1994) The effects of energy source and tryptophan on the rate of protein synthesis and on hormones of the entero-insular axis in the piglet. Br J Nutr 71(5):661–674
Schaart MW, Schierbeek H, van der Schoor SRD, Stoll B, Burrin DG, Reeds PJ, van Goudoever JB (2005) Threonine utilization is high in the intestine of piglets. J Nutr 135(4):765–770
Le Floc’h N, Seve B (2005) Catabolism through the threonine dehydrogenase pathway does not account for the high first-pass extraction rate of dietary threonine by the portal drained viscera in pigs. Br J Nutr 93(4):447–456. doi:10.1079/bjn20051375
Chen Y, Li DF, Dai ZL, Piao XS, Wu ZL, Wang B, Zhu YH, Zeng ZK (2014) l-Methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids 46(4):1131–1142. doi:10.1007/s00726-014-1675-5
Conde-Aguilera JA, Cobo-Ortega C, Tesseraud S, Mercier Y, van Milgen J (2014) The amino acid composition of tissue protein is affected by the total sulfur amino acid supply in growing pigs. Animal 8(3):401–409. doi:10.1017/S1368980008002541
Marion J, Biernat M, Thomas F, Savary G, Le Breton Y, Zabielski R, Le Huerou-Luron I, Le Dividich J (2002) Small intestine growth and morphometry in piglets weaned at 7 days of age. Effects of level of energy intake. Reprod Nutr Dev 42(4):339–354. doi:10.1051/rnd:2002030
Hamard A, Seve B, Le Floc’h N (2007) Intestinal development and growth performance of early-weaned piglets fed a low-threonine diet. Animal 1(8):1134–1142. doi:10.1017/S1751731107000560
Boudry G, Peron V, Le Huerou-Luron I, Lalles JP, Seve B (2004) Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J Nutr 134(9):2256–2262
Kansagra K, Stoll B, Rognerud C, Niinikoski H, Ou CN, Harvey R, Burrin D (2003) Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol 285(6):G1162–G1170. doi:10.1152/ajpgi.00243.2003
Aw TY (1999) Molecular and cellular responses to oxidative stress and changes in oxidation-reduction imbalance in the intestine. Am J Clin Nutr 70(4):557–565
Bauchart-Thevret C, Stoll B, Burrin DG (2009) Intestinal metabolism of sulfur amino acids. Nutr Res Rev 22(2):175–187. doi:10.1017/S0954422409990138
Jonas CR, Ziegler TR, Gu LH, Jones DP (2002) Extracellular thiol/disulfide redox state affects proliferation rate in a human colon carcinoma (Caco2) cell line. Free Radic Biol Med 33(11):1499–1506
Noda T, Iwakiri R, Fujimoto K, Aw TY (2001) Induction of mild intracellular redox imbalance inhibits proliferation of CaCo-2 cells. FASEB J 15(12):2131–2139. doi:10.1096/fj.01-0131com
Lash LH (2011) Renal membrane transport of glutathione in toxicology and disease. Vet Pathol 48(2):408–419. doi:10.1177/0300985810375811
Anderson ME, Meister A (1980) Dynamic state of glutathione in blood plasma. J Biol Chem 255(20):9530–9533
Griffith OW, Meister A (1979) Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci USA 76(11):5606–5610
Morand C, Rios L, Moundras C, Besson C, Remesy C, Demigne C (1997) Influence of methionine availability on glutathione synthesis and delivery by the liver. J Nutr Biochem 8(5):246–255. doi:10.1016/s0955-2863(97)89661-1
Richie JP, Komninou D, Leutzinger Y, Kleinman W, Orentreich N, Malloy V, Zimmerman JA (2004) Tissue glutathione and cysteine levels in methionine-restricted rats. Nutrition 20(9):800–805. doi:10.1016/j.nut.2004.05.009
Lu SC (2009) Regulation of glutathione synthesis. Mol Aspects Med 30(1–2):42–59. doi:10.1016/j.mam.2008.05.005
Miller LT, Watson WH, Kirlin WG, Ziegler TR, Jones DP (2002) Oxidation of the glutathione/glutathione disulfide redox state is induced by cysteine deficiency in human colon carcinoma HT29 cells. J Nutr 132(8):2303–2306
Perez-Vilar J, Hill R (2004) Mucin family of glycoproteins. In: Lennarz WJ, Lane E (eds) Encyclopedia of biological chemistry. Academic Press/Elsevier, Oxford, pp 758–764
Van Klinken BJ, Dekker J, Buller HA, de Bolos C, Einerhand AW (1997) Biosynthesis of mucins (MUC2-6) along the longitudinal axis of the human gastrointestinal tract. Am J Physiol 273(2 Pt 1):G296–G302
Acknowledgments
This research was supported by the Rhodimet Research Grant 2010 from Adisseo SAS (France). The authors gratefully acknowledge Nadine Mézière, Colette Mustière, Yolande Jaguelin, Armelle Cahu, Michèle Formal, Jean Noël Thibault and Philippe Ganier for their excellent assistance with sampling and laboratory analyses, Alain Chauvin, Regis Janvier, Francis Le Gouevec and Vincent Piedvache, for surgical procedures and animal care, Maurice Alix, Georges Guillemois, Jérôme Liger and Jean-François Rouaud for diet preparation and slaughter procedures (INRA, Saint-Gilles, France). J.A.C-A., N.L.F., I.LH-L., L.L., Y.M., S.T. and J.V.M. designed and conducted the research, wrote the paper and had primary responsibility for the final content; J.A.C-A. analyzed the data. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Conde-Aguilera, J.A., Le Floc’h, N., Le Huërou-Luron, I. et al. Splanchnic tissues respond differently when piglets are offered a diet 30 % deficient in total sulfur amino acid for 10 days. Eur J Nutr 55, 2209–2219 (2016). https://doi.org/10.1007/s00394-015-1031-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1031-x