Abstract
Background
The imbalance of n-6 and n-3 polyunsaturated fatty acids in the maternal diet impairs intestinal barrier development and sensitizes the colon response to inflammatory insults in the young rats. With a view to overcoming this issue, we designed this study to investigate the effect of maternal and neonatal intake of different proportions of n-6/n-3 fatty acids on colon inflammation in the young adult rats.
Methods
Female Wistar rats were assigned into four groups, and each group fed one of four semisynthetic diets, namely n-6, low n-3, n-6/n-3 and n-3 fatty acids for 8 weeks prior to mating, during gestation and lactation periods. At weaning, the pups were separated from the dams and fed diet similar to the mothers. Colitis was induced on postnatal day 35, by administering 2 % dextran sulfate sodium in drinking water for 10 days. Colitis was assessed based on the clinical and inflammatory markers in the colon. Fatty acid analysis was done in liver, RBC, colon and spleen.
Results
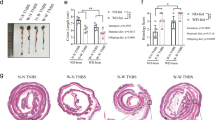
A balanced n-6/n-3 PUFA diet significantly improved the body weight loss, rectal bleeding and mortality in rats. This was associated with lower myeloperoxidase activity, nitric oxide, prostaglandin E2, TNF-α and IL-6, IL-8, COX-2 and iNOS levels in the colon tissues. Fatty acid analysis has shown that the arachidonic acid/docosahexaenoic acid ratio was significantly lower in liver, RBC, colon and spleen in n-6/n-3 and n-3 diet groups.
Conclusion
We demonstrate that balanced n-6/n-3 PUFA supplementation in maternal and neonatal diet alters systemic AA/DHA ratio and attenuates colon inflammation in the young adult rats.












Similar content being viewed by others
Abbreviations
- MUFA:
-
Monounsaturated fatty acids
- PUFA:
-
Polyunsaturated fatty acids
- LA:
-
Linoleic acid
- ALA:
-
α-Linolenic acid
- AA:
-
Arachidonic acid
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- LC-PUFA:
-
Long-chain polyunsaturated fatty acids
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- COX-2:
-
Cyclooxygenase-2
- iNOS:
-
Inducible nitric oxide synthase-2
- PGE2 :
-
Prostaglandin E2
- LTB4 :
-
Leukotriene B4
- RBC:
-
Red blood cells
References
Strober W, Fuss IJ, Blumberg RS (2002) The immunology of mucosal models of inflammation. Annu Rev Immunol 20:495–549
Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease. Nature 448:427–434
Araki Y, Sugihara H, Hattori T (2006) The free radical scavengers edaravone and tempol suppress experimental dextran sulphate sodium induced colitis in mice. Int J Mol Med 17:331–334
Kolios G, Valatas V, Ward SG (2004) Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology 113:427–437
Bonner GF (2002) Using COX-2 inhibitors in IBD: anti-inflammatories inflame a controversy. Am J Gastroenterol 97:783–785
Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF (1998) Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 115:297–306
Rojas-Cartagena C, Flores I, Appleyard CB (2005) Role of tumor necrosis factor receptors in an animal model of acute colitis. Cytokine 32:85–93
Atreya R, Neurath MF (2005) Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol 28:187–196
Mitsuyama K, Toyonaga A, Sasaki E, Watanabe K, Tateishi H, Nishiyama T, Tanikawa K (1994) IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn’s disease. Clin Exp Immunol 96:432–440
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Calder PC (2008) Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 52:1–13
Arterburn LM, Hall EB, Oken H (2006) Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr 83:1467S–1476S
Barcelo-Coblijn G, Murphy EJ (2009) Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog Lipid Res 48(6):355–374
Emken EA, Adlof RO, Gulley RM (1994) Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta 1213:277–288
Vermunt SH, Mensink RP, SimonisMM Hornstra G (2000) Effects of dietary alpha-linolenic acid on the conversion and oxidation of 13C-alpha linolenic acid. Lipids 35:137–142
Kevan J, Mundra H, Innis SM (2005) Intestinal responsiveness to experimental colitis in the young rats is altered by maternal diet. Am J Physiol Gastrointest Liver Physiol 289:13–20
Innis M, Jacobson K (2007) Dietary lipids in early development and intestinal inflammatory disease. Nutr Rev 65:S188–S193
Berthold K, Eric L, Carlo A, Hansjosef B, Cristina C, Irene C, Tamas D, Joachim WD, Cristophe D, Stewart F, Irene H, Wolfgang H, Alexandre L, Guy P, Niels JS, Mike S, Hania S, Peter W, Ricardo U (2008) The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 36:5–14
Diwakar BT, Dutta PK, Lokesh BR, Naidu KA (2010) Physicochemical properties of garden cress (Lepidium sativum L.) seed oil. J Am Oil Chem Soc 87:539–548
Reddy KVK, Maheswaraiah A, Naidu KA (2014) Rice bran oil and garden cress (Lepidium sativum L.) seed oil attenuate murine model of colitis. Int J Colorectal Dis 29:267–269
Islam MS, Murata T, Fujisawa M, Nagasaka R, Ushio H, Bari AM, Hori M, Ozaki H (2008) Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br J Pharmacol 154:812–824
Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK (2003) Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol 139:209–218
Tsai WC, Strieter RM, Zisman DA, Wilkowski JM, Bucknell KA, Chen G, Standiford TJ (1997) Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun 65:1870–1875
Piazza GA, Alberts DS, Hixson LJ, Paranka NS, Li H, Finn T, Bogert C, Guillen JM, Brendel K, Gross PH, Sperl G, Ritchie R, Burt RW, Ellsworth L, Ahnen DJ, Pamukcuet R (1997) Sulindac sulfone inhibits azoxymethane-induced colon carcinogenesis in rats without reducing prostaglandin levels. Cancer Res 57:2909–2915
Folch J, Lees M, Sloane-Stanley GH (1956) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron trifluoride methanol. J Lipid Res 5:600–608
Barcelo CG, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK (2008) Flaxseed oil and fish-oil capsule consumption alters human red blood cell n–3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n–3 fatty acid. Am J Clin Nutr 88:801–809
Krawisz JE, Sharon P, Stenson WF (1984) Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: assessment of inflammation in rat and hamster models. Gastroenterology 84:1344–1350
Charles SN, Savill J (2005) Resolution of inflammation: the beginning programs the end. Nat Immunol 6:1191–1197
Mazzucchelli L, Hauser C, Zgraggen K, Wagner H, Hess M, Laissue JA, Mueller C (1994) Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol 144:997–1007
Tapiero H, Nguyen Ba G, Couvreur P, Tew KD (2002) Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother 56:215–222
Carty E, DeBrabander M, Feakins RM, Rampton DS (2000) Measurement of in vivo rectal mucosal cytokine and eicosanoid production in ulcerative colitis using filter paper. Gut 46:487–492
Malkoswki MG, Thuresson ED, Lakkides KM, Rieke J, Micielli R, Smith WL et al (2001) Structure of eicosapentaenoic and linolenic acids in the cyclooxygenase site of prostaglandin endoperoxide H synthase-1. J Biol Chem 40:37547–37555
Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L et al (2004) Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem 279:16971–16979
Pryor WA, Squadrito GL (1995) The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol 268:L699–L722
Cross RK, Wilson KT (2003) Nitric oxide in inflammatory bowel disease. Inflamm Bowel Dis 9:179–189
Kelley DS, Taylor PC, Nelson GJ et al (1999) Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids 34:317–324
Camuesco D, Comalada M, Concha A, Nieto A, Sierra S, Xaus J, Zarzuelo A, Galvez J (2006) Intestinal anti-inflammatory activity of combined quercetin and dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, in rats with DSS-induced colitis. Clin Nutr 25:466–476
Komatsu W, Ishihara K, Murata M, Saito H, Shinohara K (2003) Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-γ plus lipopolysaccharide stimulated murine macrophages by inhibiting the oxidative stress. Free Radic Biol Med 34:1006–1016
Thomsen MK, Larsen CG, Thomsen HK, Kirstein D, Skak-Nielsen T, Ahnfelt-Rønne I, Thestrup-Pedersen K (1991) Recombinant human interleukin-8 is a potent activator of canine neutrophil aggregation, migration, and leukotriene B4 biosynthesis. J Invest Dermatol 62:260–266
Daig R, Andus T, Aschenbrenner E, Falk W, Schölmerich J, Gross V (1996) Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut 38:216–222
Malsushima K, Monshita K, Yoshimura T et al (1988) Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumour necrosis factor. J Exp Med 167:1883–1893
Dey I, Giembycz MA, Chadee K (2009) Prostaglandin E(2) couples through EP(4) prostanoid receptors to induce IL-8 production in human colonic epithelial cell lines. Br J Pharmacol 156:475–485
Storey A, McArdle F, Peter S, Friedmann W, Jackson MJ, Rhodesz LE (2005) Eicosapentaenoic Acid and Docosahexaenoic Acid Reduce UVB- and TNF-α induced IL-8 secretion in keratinocytes and UVB-induced IL-8 in fibroblasts. J Invest Dermatol 124:248–255
Babcock TA, Novak T, Ong E, Jho DH, Helton WS, Espat NJ (2002) Modulation of lipopolysaccharide-stimulated macrophage tumor necrosis factor-α production by n-3 fatty acid is associated with differential cyclooxygenase-2 protein expression and is independent of interleukin-10. J Surg Res 107:135–139
Zhao Y, Joshi-Barve S, Barve S, Chen LH (2004) Eicosapentaenoic acid prevents LPS-induced TNF-α expression by preventing NF-kB activation. J Am Coll Nutr 23:71–78
Yaqoob P, Calder PC (1995) Effects of dietary lipid manipulation upon inflammatory mediator production by murine macrophages. Cell Immunol 163:120–128
Renier G, Skamene E, de Sanctis J, Radzioch D (1993) Dietary n-3 polyunsaturated fatty acids prevent the development of atherosclerotic lesions in mice: modulation of macrophage secretory activities. Arterioscler Thomb 13:1515–1524
Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ (1996) The effect on human tumor necrosis factor-α and interleukin-1β production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr 63:116–122
Trebble T, Arden NK, Stroud MA et al (2003) Inhibition of tumour necrosis factor-α and interleukin-6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br J Nutr 90:405–412
Pawlosky RJ, Hibblen JR, Lin Y et al (2003) Effects of beef- and fish-based diets on the kinetics of n-3 fatty acid metabolism in human subjects. Am J Clin Nutr 77:565–572
Goyens PL, Spilker ME, Zock PL, Katan MB, Mensink RP (2005) Compartmental modelling to quantify alpha-linolenic acid conversion after longer-term intake of multiple tracer boluses. J Lipid Res 46:1474–1483
Innis SM, Dai C, Wu X, Buchan AMJ, Jacobson K (2010) Perinatal lipid nutrition alters early intestinal development and programs the response to experimental colitis in young adult rats. Am J Physiol Gastrointest Liver Physiol 299:1376–1385
Martin-Venegas R, Roig-Perez S, Ferrer R, Moreno JJ (2006) Arachidonic acid cascade and epithelial barrier function during Caco-2 cell differentiation. J Lipid Res 47:1416–1423
Morteau O, Morham SG, Sellon R, Dieleman L, Langenbach R, Smithies O, Sartor RB (2000) Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest 105:469–478
Acknowledgments
The authors thank Director, Prof. Ram Rajasekharan, CSIR-CFTRI, Mysore, for his support and encouragement in the present study. Mr. K.V.K Reddy, CSIR-Senior Research Fellow gratefully acknowledges the financial assistance from Council of Scientific and Industrial Research (CSIR), New Delhi, in carrying out these investigations. KAN gratefully acknowledges the financial support in the form of a Project (SR/SO/HS-0005/2010) awarded by Department of Science and Technology (DST), New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors do not have any conflict of interest.
Rights and permissions
About this article
Cite this article
Reddy, K.V.K., Naidu, K.A. Maternal and neonatal dietary intake of balanced n-6/n-3 fatty acids modulates experimental colitis in young adult rats. Eur J Nutr 55, 1875–1890 (2016). https://doi.org/10.1007/s00394-015-1004-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1004-0