Abstract
Objective
This study systemically reviewed the evidence regarding the association between plasminogen activator inhibitor‑1 (PAI‑1) 4G/5G polymorphism and susceptibility to systemic lupus erythematous (SLE)/lupus nephritis (LN) and rheumatoid arthritis (RA) and the relationship between circulating PAI‑1 levels and SLE/LN and RA.
Methods
We conducted a meta-analysis on the association between the PAI‑1 4G/5G polymorphism and SLE/LN or RA risk and serum/plasma PAI‑1 levels in patients with SLE/LN and RA and healthy controls.
Results
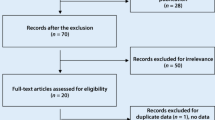
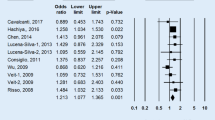
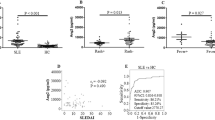
Nine articles including 657 patients with SLE and 668 controls and 567 patients with RA and 772 controls were included. No association was revealed between SLE and PAI‑1 4G allele in all study subjects (odds ratio [OR] = 0.944, 95% confidence interval [CI] = 0.808–1.102, p = 0.463). Ethnicity-based stratification showed no association between the PAI‑1 4G allele and SLE among Europeans and Asians. No association was detected between LN and RA and the PAI‑1 4G allele (OR = 0.886, 95% CI = 0.713–1.102, p = 0.278; OR = 0.8736, 95% CI = 0.747–1.020, p = 0.088, respectively) or between SLE/LN and RA and the PAI‑1 4G/5G polymorphism using the recessive and dominant models and homozygote contrast. The circulating PAI‑1 level was significantly higher in the SLE group than in the control group (standardized mean difference [SMD] = 0.337, 95% CI = 0.057–0.619, p = 0.019). However, serum/plasma PAI‑1 level showed no significant difference between RA and control group (SMD = 0.333, 95% CI = −0.6989–1.35, p = 0.527).
Conclusions
There was no association between the PAI‑1 4G/5G polymorphism and SLE/LN and RA development and significantly higher levels of circulating PAI‑1 were observed in patients with SLE but not in those with RA.
Zusammenfassung
Zielsetzung
In der Studie wurde systematisch die Evidenz für einen Zusammenhang zwischen dem PAI‑1(Plasminogenaktivator-Inhibitor)-4G/5G-Polymorphismus und der Suszeptibilität für systemischen Lupus erythematous (SLE)/Lupusnephritis (LN) bzw. rheumatoide Arthritis (RA) und für eine Beziehung zwischen der Höhe an zirkulierendem PAI‑1 und SLE/LN bzw. RA untersucht.
Methoden
Wir führten eine Metaanalyse durch zum Zusammenhang zwischen PAI-1-4G/5G-Polymorphismus und SLE/LN- bzw. RA-Risiko sowie zwischen Serum/Plasma-PAI-1-Level bei Patienten mit SLE/LN und RA sowie gesunden Kontrollen.
Ergebnisse
Neun Publikationen (657 SLE-Patienten, 668 Kontrollen; 567 RA-Patienten, 772 Kontrollen) wurden in die Metaanalyse aufgenommen. Es zeigte sich kein Zusammenhang zwischen SLE und PAI-1-4G-Allel bei allen Teilnehmenden (Odds Ratio [OR] 0,944, 95 %-Konfidenzintervall [KI] 0,808–1,102; p = 0,463). Eine Stratifizierung nach Ethnizität zeigte keinen Zusammenhang zwischen dem PAI-1-4G-Allel und SLE bei Europäern und Asiaten, ebenso bestand keiner zwischen LN bzw. RA und dem PAI-1-4G-Allel (OR 0,886, 95 %–KI 0,713‑1,102, p = 0,278; OR 0,8736, 95 %-KI 0,747‑1,020, p = 0,088) bzw. zwischen SLE/LN bzw. RA und dem PAI-1-4G/5G-Polymorphismus unter Verwendung der rezessiven und dominanten Modelle und des „homozygote contrast“. Der zirkulierende PAI-1-Wert war in der SLE-Gruppe signifikant höher als in der Kontrollgruppe (standardisierte mittlere Differenz [SMD] 0,337, 95 %-KI 0,057–0,619, p = 0,019), doch der PAI-1-Spiegel im Serum/Plasma wies keinen signifikanten Unterschied auf zwischen RA- und Kontrollgruppe (SMD 0,333, 95 %-KI -0,6989–1,35, p = 0,527).
Schlussfolgerungen
Es gab keinen Zusammenhang zwischen dem PAI-1-4G/5G-Polymorphismus und SLE/LN- bzw. RA-Entwicklung. Signifikant erhöhte Level an zirkulierendem PAI‑1 wurden bei SLE-, nicht aber bei RA-Patienten beobachtet.


Similar content being viewed by others
References
Arlestig L, Wallberg Jonsson S, Stegmayr B et al (2007) Polymorphism of genes related to cardiovascular disease in patients with rheumatoid arthritis. Clin Exp Rheumatol 25(6):866–871
Bicakcigil M, Tasan D, Tasdelen N et al (2011) Role of fibrinolytic parameters and plasminogen activator inhibitor 1 (PAI-1) promoter polymorphism on premature atherosclerosis in SLE patients. Lupus 20(10):1063–1071
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Erlbaum, Hillsdale, New Jersey
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Egger M, Smith GD, Phillips AN (1997) Meta-analysis: principles and procedures. BMJ 315(7121):1533–1537
Eriksson P, Kallin B, van ’t Hooft FM et al (1995) Allele-specific increase in basal transcription of the plasminogen-activator inhibitor 1 gene is associated with myocardial infarction. Proc Natl Acad Sci U S A 92(6):1851–1855
Festa A, D’Agostino R Jr, Rich SS et al (2003) Promoter (4G/5G) plasminogen activator inhibitor‑1 genotype and plasminogen activator inhibitor‑1 levels in blacks, Hispanics, and non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Circulation 107(19):2422–2427
Gong R, Liu Z, Li L (2007) Epistatic effect of plasminogen activator inhibitor 1 and beta-fibrinogen genes on risk of glomerular microthrombosis in lupus nephritis: interaction with environmental/clinical factors. Arthritis Rheum 56(5):1608–1617
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Hoppe B, Dörner TJNRR (2012) Coagulation and the fibrin network in rheumatic disease: a role beyond haemostasis. Nat Rev Rheumatol 8(12):738–746
Hoppe B, Häupl T, Skapenko A et al (2012) Fibrinogen and factor XIII A‑subunit genotypes interactively influence C‑reactive protein levels during inflammation. Ann Rheum Dis 71(7):1163–1169
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Lee YH, Bae S‑C, Choi SJ et al (2012) Associations between TNFAIP3 gene polymorphisms and rheumatoid arthritis: a meta-analysis. Inflamm Res 61(6):635–641
Lee YH, Bae S‑C, Choi SJ et al (2012) Genome-wide pathway analysis of genome-wide association studies on systemic lupus erythematosus and rheumatoid arthritis. Mol Biol Rep 39(12):10627–10635
Lee YH, Bae SC, Song GG (2013) Hepatitis B virus reactivation in HB s A g‑positive patients with rheumatic diseases undergoing anti-tumor necrosis factor therapy or DMARD s. Int J Rheum Dis 16(5):527–531
Lee YH, Song GG (2017) Comparative efficacy and safety of secukinumab and adalimumab in patients with active ankylosing spondylitis: A Bayesian network meta-analysis of randomized controlled trials. J Rheum Dis 24(4):211–219
Li W, Mao S, Wu L et al (2019) Association between the PAI‑1 4G/5G gene polymorphism and the risk of systemic lupus Erythematosus/lupus nephritis. Crit Rev Eukaryot Gene Expr 29(1):85–94
Ma Z, Paek D, Oh C (2009) Plasminogen activator inhibitor‑1 and asthma: role in the pathogenesis and molecular regulation. Clin Exp Allergy 39(8):1136–1144
Mellado M, Martínez-Muñoz L, Cascio G et al (2015) T cell migration in rheumatoid arthritis. Front Immunol 6:1–12
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 6(7):e1000097
Munoz-Valle JF, Ruiz-Quezada SL, Oregon-Romero E et al (2012) PAI‑1 mRNA expression and plasma level in rheumatoid arthritis: relationship with 4G/5G PAI‑1 polymorphism. Rheumatol Int 32(12):3951–3956
Munoz SA, Aranda F, Allievi A et al (2014) 4G/5G plasminogen activator inhibitor‑1 and -308 A/G tumor necrosis factor-alpha promoter gene polymorphisms in Argentinean lupus patients: focus on lupus nephritis. Clin Exp Med 14(1):83–89
Reshetniak TM, Ostriakova EV, Patrusheva NL et al (2013) Plasminogen activator inhibitor type 1 gene polymorphism and thromboses in patients with antiphospholipid syndrome. Ter Arkh 85(1):76–84
Ridout KK, Ridout SJ, Price LH et al (2016) Depression and telomere length: a meta-analysis. J Affect Disord 191:237–247
Ruiz-Irastorza G, Khamashta MA, Castellino G et al (2001) Systemic lupus erythematosus. Lancet 357(9261):1027–1032
Singh NK, Gupta A, Behera DR et al (2013) Elevated plasminogen activator inhibitor type‑1 (PAI-1) as contributing factor in pathogenesis of hypercoagulable state in antiphospholipid syndrome. Rheumatol Int 33(9):2331–2336
Sprengers ED, Kluft C (1987) Plasminogen activator inhibitors. Blood 69(2):381–387
Stegnar M, Uhrin P, Peternel P et al (1998) The 4G/5G sequence polymorphism in the promoter of plasminogen activator inhibitor‑1 (PAI-1) gene: relationship to plasma PAI‑1 level in venous thromboembolism. Thromb Haemost 79(5):975–979
Tassies D, Espinosa G, Munoz-Rodriguez FJ et al (2000) The 4G/5G polymorphism of the type 1 plasminogen activator inhibitor gene and thrombosis in patients with antiphospholipid syndrome. Arthritis Rheum 43(10):2349–2358
Wang AY, Poon P, Lai FM et al (2001) Plasminogen activator inhibitor‑1 gene polymorphism 4G/4G genotype and lupus nephritis in Chinese patients. Kidney Int 59(4):1520–1528
Acknowledgements
This study was supported in part by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (NRF-2017M3A9B4050335), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.-C. Bae and Y. H. Lee declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
Additional information
Redaktion
U. Müller-Ladner, Bad Nauheim
U. Lange, Bad Nauheim
Rights and permissions
About this article
Cite this article
Bae, SC., Lee, Y.H. Association between plasminogen activator inhibitor‑1 (PAI-1) 4G/5G polymorphism and circulating PAI-1 level in systemic lupus erythematosus and rheumatoid arthritis. Z Rheumatol 79, 312–318 (2020). https://doi.org/10.1007/s00393-019-00689-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-019-00689-y
Keywords
- Matrix metalloproteinases
- Serine proteinase inhibitors
- Polymorphism, genetic
- Autoimmune diseases
- Case-control studies