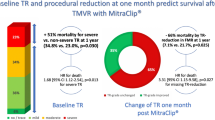
Abstract
Background
To assess the interaction between heart failure (HF) severity and optimal reduction of secondary mitral regurgitation (SMR) on mortality in patients undergoing transcatheter edge-to-edge repair (M-TEER).
Methods and results
Among 1656 patients included in the Italian Society of Interventional Cardiology (GIse) registry Of Transcatheter treatment of mitral valve regurgitaTiOn (GIOTTO) 984 had SMR and complete data on advanced HF. Advanced HF was defined as NYHA class III or IV, left ventricular ejection fraction ≤ 30%, and > 1 HF hospitalization during the last 12 months. Optimal M-TEER was defined as residual SMR ≤ 1 + at discharge. One hundred sixteen patients (11.8%) had advanced HF. Achievement of an optimal SMR reduction was similar in patients with and without advanced HF (65% and 60% respectively). Advanced HF was an independent predictor of 2-year all-cause death (adjusted HR 1.52, 95% CI 1.09–2.10). Optimal M-TEER, as compared to a no-optimal M-TEER, was associated with a reduced risk of death both in patients with advanced (HR 0.55, 95% CI 0.32–0.97; p = 0.039) and no-advanced HF (HR 0.59, 95% CI 0.46–0.78; p < 0.001; p = 0.778 for interaction).
Conclusions
Advanced HF is associated with poor outcome in patients undergoing M-TEER. However, an optimal SMR reduction reduces the risk of 2-year mortality regardless of HF severity.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary mitral regurgitation (SMR) is common in patients with chronic heart failure (HF) and it is known to be associated with poor outcome [1, 2]. Current guidelines recommend mitral transcatheter edge-to-edge repair (M-TEER), with a class IIa recommendation, for HF patients with significant SMR and the specific clinical and echocardiographic characteristic [3, 4] of the patients enrolled in the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) trial [5, 6]. Conversely, the indication to M-TEER is currently IIb, as an alternative to palliative care or as a bridge to other therapies, for the patients with HF who do not fulfill the COAPT criteria [3]. Notably, advanced HF was a major exclusion criterion in COAPT.
Nevertheless, some registries reported a possible beneficial effect of M-TEER in the setting of advanced HF showing both quality of life and symptoms improvement after the procedure [7,8,9,10,11,12,13,14].
Furthermore, M-TEER has been reported as a possible bridge strategy to left ventricular assist device or heart transplantation with a relevant proportion of patients improving their hemodynamic conditions after SMR reduction [15, 16]. However, it is currently unknown whether M-TEER may have an impact on mortality in patients with advanced HF.
The aim of this analysis is to explore the role of advanced HF in patients undergoing M-TEER as well as the association between an optimal reduction of SMR by means of M-TEER and mortality in patients with and without advanced HF.
Methods
Population and definitions
The multicenter Italian Society of Interventional Cardiology (GIse) registry Of Transcatheter treatment of mitral valve regurgitaTiOn (GIOTTO) is a single-arm, multicenter, prospective registry conceived to collect data regarding patients with symptomatic mitral regurgitation (MR) who underwent MitraClip between 2016 and 2020, reflecting standard clinical practice in Italian hospitals. Qualifying inclusion and exclusion criteria, echocardiographic selection and protocols employed, and details of the MitraClip procedure have been reported previously [17].
For the purpose of the present analysis, we only included patients with SMR and available data on advanced HF. The population of interest was stratified according to the presence of advanced HF.
Based on recent recommendations, advanced HF was defined according to the following criteria (all must be fulfilled): NYHA functional class III or IV, LVEF ≤ 30%, and > 1 hospitalization for HF during the last 12 months [3, 18]. Of note, information regarding baseline functional status (i.e., 6 min walking test and pulmonary exercise test) was not available in the GIOTTO registry.
Optimal M-TEER was defined as mild or less residual SMR (MR ≤ 1 +) assessed at discharge.
Outcome of interest was all-cause mortality assessed at 2-year follow-up. Additional outcomes were HF hospitalization and the composite of all-cause mortality and HF hospitalization.
Statistical analysis
Categorical and dichotomous variables were expressed as absolute numbers and percentages and were compared by the chi-square test. Continuous variables were expressed as mean ± standard deviation, or median and interquartile range (25th–75th IQR), as appropriate. Unpaired Student’s t-test was used to compare continuous parameters following a normal distribution, while the Mann–Whitney U test was used to compare continuous variables with skewed distribution.
Two-year follow-up Kaplan–Meier curves for all-cause mortality, HF hospitalization, and the composite of all-cause mortality and HF hospitalization were computed after stratification by the presence of advanced HF. Comparisons were made with the log-rank test.
Univariate and multivariate Cox proportional hazards regression was used to assess the association between advanced HF and outcomes and to estimate corresponding hazard ratios (HR) and 95% confidence intervals (CI).
Variables included in the multivariable model were EuroSCORE II, chronic obstructive pulmonary disease (COPD), coronary artery disease (CAD), glomerular filtration rate (GFR), left ventricle end-systolic volume (LVESV), tricuspid annular plane systolic excursion (TAPSE), and optimal M-TEER.
Interaction between optimal M-TEER and advanced HF on outcomes was tested with a Cox regression analysis and reported as p for interaction.
For all analyses, the SPSS statistics software (version 25, IBM Corp., Armonk, NY, USA) was used and a p-value of ≤ 0.05 was set for significance.
Results
Baseline and procedural data
Among 1656 patients included in the GIOTTO registry, 984 had SMR and complete information on advanced HF Among them, 116 (11.8%) were classified as having advanced HF and 868 (88.2%) as having no-advanced HF (Fig. 1). Overall, 896 (91%) of patients had at least 1 criterion included in the definition of advanced HF. Distribution of advanced HF criteria is reported in Fig. 2. Baseline characteristics of the overall population, and stratified by the presence of advanced HF, are reported in Table 1.
Patients with advanced HF were younger and with a lower body mass index (BMI) as compared to those without advanced HF. In addition, they were more likely to have chronic obstructive pulmonary disease (COPD), higher EuroSCORE II, poorer kidney function, larger left ventricle, and worse left and right ventricular function, compared with the others. Regarding medical therapy, there were no differences in the proportion of patients receiving renin-angiotensin system antagonists or beta-blockers. However, compared with patients with no-advanced HF, those with advanced HF more frequently received loop diuretics and mineralocorticoid receptor antagonist (MRA).
Procedural and discharge data are shown in Table 2. An optimal SMR reduction, assessed at discharge, was achieved in 60% of patients with advanced HF and in 65% of patients with no-advanced HF (p = 0.304) (Table 2 and Fig. 1). Procedural time as well as device time was longer in patients with as compared to those without advanced HF. No differences were noted between the two groups in terms of number of clips deployed and mean pressure gradient at discharge.
Outcomes
Median follow-up was 614 days (IQ 317–763). At 2 years, 268 patients died. As expected, cumulative incidence of all-cause death was higher in patients with advanced HF versus those without advanced HF (47% vs 29%) (Fig. 3). Advanced HF was a predictor of death at both univariate (HR 1.75, 95% CI 1.29–2.39; p < 0.001) and multivariable (HR 1.52, 95% CI 1.09–2.1; p = 0.010) analyses. An optimal M-TEER was associated with a lower cumulative incidence of 2-year all-cause death, as compared to a no-optimal M-TEER, in both advanced (40% vs 57%; HR 0.55, 95% CI 0.32–0.97; p = 0.039) and non-advanced (24% vs 38%; HR 0.59, 95% CI 0.46–0.78; p < 0.001) HF patients (Fig. 4). Advanced HF did not affect the association between optimal M-TEER and all-cause death (p for interaction, 0.736).
Likewise, advanced HF was associated with higher incidence of HF hospitalization (HR 2.085, CI 1.393–3.120, p < 0.001) and of the composite outcome of all-cause mortality and HF hospitalization (HR 2.080, CI 1.529–2.829, p < 0.001) (Supplementary Figs. 1 and 2). An optimal M-TEER was associated with a lower incidence of HF hospitalization (HR 0.555, CI 0.392–0.784, p = 0.001) and of all-cause mortality and HF hospitalization (HR 0.603, CI 0.461–0.788, p < 0.001) in non-advanced HF (Supplementary Figs. 3 and 4), but not in advanced HF patients (HR 0.969, CI 0.457–2.054, p = 0.934 and HR 0.627, CI 0.379–1.040, p = 0.070 respectively) (Supplementary Figs. 5 and 6). However, advanced HF did not affect the association between optimal M-TEER and additional outcomes (p for interaction 0.171 for HF hospitalization, and 0.778 for all-cause mortality and HF hospitalization).
Discussion
The main findings of the present study are the following: i) among patients with SMR undergoing M-TEER in the GIOTTO multicenter registry, those with advanced HF had a higher risk of mortality and, ii) an optimal M-TEER was associated with a lower risk of mortality, as compared to a no-optimal M-TEER, in both patients with and without advanced HF.
M-TEER is a well-established therapy for patients with significant isolated SMR and favorable clinical and echocardiographic characteristics, as in the COAPT Trial [6, 19, 20]. However, a high proportion of real-world patients has a non-COAPT profile [13, 21] and, despite the expanded use of M-TEER also in this setting, [22] evidence supporting this indication is limited. Specifically, some registries reported safety and feasibility of M-TEER in non-COAPT patients as well as in patients with advanced or severe HF with an improvement of hemodynamic parameters as well as symptoms, functional status, and quality of life after the procedure [7, 10, 16]. However, a comparison group (i.e., patients who did not receive the intervention) was missing in previous studies and data regarding the effective prognostic benefit of M-TEER in patients with advanced HF are lacking.
Also, data regarding the natural history and prognostic role of severe SMR in the setting of advanced HF are limited. A recent study showed that severe SMR was associated with an increased risk of cardiovascular mortality and recurrent HF hospitalization, but not all-cause mortality in patients with advanced HF [23]. Furthermore, in current guidelines, the hypothetical goal of the indication for M-TEER in non-COAPT patients is the improvement of HF symptoms alone.
Prognostic role of advanced HF in M-TEER
Several registries underlined the negative prognostic role of variables related to advanced HF in patients undergoing M-TEER [7, 24,25,26,27]. Predictors of 1-year mortality in the German multicenter TRAnscatheter Mitral valve Interventions (TRAMI) registry were NYHA class IV (HR 1.62), anemia (HR 2.44), renal failure with serum creatinine ≥ 1.5 mg/dL (HR 1.77), LVEF < 30% (HR 1.59), and severe tricuspid regurgitation (HR 1.84) [28]. In this registry, among 777 patients undergoing MitraClip, 256 (33.0%) had a LVEF < 30% [11, 28]. Multivariable analysis revealed an increased risk of major adverse events (mainly driven by all-cause death) in patients with severely impaired LV function. Advanced NYHA class, especially NYHA IV, was extensively reported as associated with poorer outcome even after M-TEER [27,28,29]. Franzen et al. [7] showed that among patients with SMR and advanced HF, NYHA class, elevated NTpro-BNP levels, and LV dimensions were major predictors of adverse outcome [7]. Recurrent (> 1) HF hospitalizations before M-TEER have also been reported as associated with unfavorable outcome after the procedure [30].
We confirm all these previous findings showing that advanced HF, defined as NYHA III or IV, LVEF ≤ 30%, and > 1 HF hospitalization, was associated with an increased risk of 2-year all-cause mortality (regardless of possible confounders), HF hospitalization, and the composite of all-cause mortality and HF hospitalization. Notably, right ventricular (RV) function has a crucial prognostic role in HF patients with SMR undergoing M-TEER [12, 31,32,33]. In our analysis, according to recent recommendations, RV function was not included in the definition of advanced HF. However, as expected, RV dysfunction (i.e. a low TAPSE) was more frequent in patients with advanced versus no-advanced HF.
Prognostic role of optimal M-TEER
Among patients with HF and severe SMR enrolled in the COAPT trial, a favorable outcome at 12-month follow-up was predicted only by lower serum creatinine, Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OS), and treatment assignment to M-TEER plus GDMT arm, corroborated by the 30-day change in MR, suggesting that the mechanism underlying clinical response to M-TEER is reduction of MR. In comparison with residual MR ≤ 1, MR 2 + demonstrated a trend towards mortality benefit at both unadjusted (HR, 1.34; 95% CI, 0.99 to 1.82; p = 0.056) and adjusted (HR, 1.35; 95% CI, 0.99 to 1.84; p = 0.057) analyses [34].
The association between an optimal MR reduction by M-TEER and improved survival in SMR patients has already been extensively reported [26, 35]. The Optimized Catheter Valvular Intervention (OCEAN-Mitral) registry, a prospective, multicenter registry to assess the safety and efficacy of TEER in significant MR, enrolled 2150 consecutive, symptomatic patients who underwent M-TEER in 21 Japanese institutions [36]. Among them, 1617 patients (75.2%) were deemed to have SMR. Looking at the single components that define advance HF, 63.2% of patients had a NYHA functional class III or IV, 71.7% HF experienced > 1 hospitalization within 1 year before TEER, but only 22.7% of them had an LVEF < 30%. Mortality rate at 1 year was lower in the residual MR ≤ 1 group (10.3%) than in the MR 2 + group (18.9%; p < 0.001) and MR 3 + /4 + group (16.9%; p = 0.06). However, in the presence of advanced HF, defined in this study by the presence of LV dilatation or RV dysfunction, the survival benefit of residual MR ≤ 1 over MR 2 + was no longer detectable.
In our analysis, residual MR ≤ 1 + after M-TEER is associated with lower all-cause mortality in patients with both advanced and no-advanced HF. Thus, our study suggests that even patients with advanced HF may experience a benefit on mortality with an optimal MR reduction by TEER. Even though in advanced HF patients HF hospitalization and the composite outcome are only numerically lower in patients receiving an optimal MR reduction as compared to those who did not, advanced HF status seems not to affect the beneficial effect of optimal M-TEER on outcomes. Notably, the possible underestimation of the number of HF hospitalizations in the GIOTTO registry may also have affected the results.
Different definitions of advanced HF may have contributed to the different result of our analysis as compared to the OCEAN-Mitral study.
Notably, the rate of optimal M-TEER results is increasing because of continuous improvement in M-TEER technology and in operators’ experience. Indeed, it is currently possible to treat complex anatomies with a high rate of optimal result by using new-generation devices at high-volume centers [37]. Proper pre-procedural planning is crucial, including evaluating the MR mechanism, annular diameters and area, and leaflet length. Considering a strategy involving two or more devices and selecting the appropriate device size are also important [38].
Further research is needed to better establish the role of M-TEER in patients with advanced HF. The ongoing MITRAl regurgitation treatment in ADVANCEd Heart Failure (MITRADVANCE-HF) prospective, randomized, controlled, open-label, multicenter trial (NCT05292716) will enrol 190 patients with SMR and advanced HF who will be randomly assigned, in a 1:1 ratio, to a device arm consisting of MitraClip therapy added to optimal medical therapy (OMT) or a control arm of OMT alone. A composite hierarchical end-point including all-cause death, HF events, and quality of life changes assessed by KCCQ will be explored at 3-month follow-up.
Limitations
Many limitations have to be acknowledged. First, this is an observational study with an intrinsic selection bias due to the lack of randomization. To partially overcome this issue, we compared patients with optimal M-TEER with those receiving a non-optimal M-TEER. Second, data were reported from different centers without independent adjudication of events. Thus, we used all-cause death as outcome of interest. Third, the reliability of the residual MR data could have been affected by the lack of a Core Laboratory and by the dynamic nature of SMR. Moreover, in the definition of advanced HF, data on exercise capacity were missing.
Finally, patients with SMR included in the GIOTTO registry should have been on optimal medical therapy. However, the rate of patients receiving ACE-i/ARBs/ARNIs was lower as compared with the current literature [5]. In addition, patients were recruited from 2016 to 2020, an era before establishment of SGLT2 inhibitors (SGLT2i) as a standard therapy for HFrEF patients.
Conclusions
In a large real-world SMR population undergoing M-TEER, advanced HF at baseline was associated with higher mortality at 2 years after the procedure. However, an optimal result of M-TEER was associated with a lower mortality irrespective of the presence of advanced HF at baseline.
References
Pagnesi M, Adamo M, Sama IE, Anker SD, Cleland JG, Dickstein K, Filippatos GS, Lang CC, Ng LL, Ponikowski P, Ravera A, Samani NJ, Zannad F, van Veldhuisen DJ, Voors AA, Metra M (2021) Impact of mitral regurgitation in patients with worsening heart failure: insights from BIOSTAT-CHF. Eur J Heart Fail 23:1750–1758
Pagnesi M, Adamo M, Sama IE, Anker SD, Cleland JG, Dickstein K, Filippatos GS, Inciardi RM, Lang CC, Lombardi CM, Ng LL, Ponikowski P, Samani NJ, Zannad F, van Veldhuisen DJ, Voors AA, Metra M (2022) Clinical impact of changes in mitral regurgitation severity after medical therapy optimization in heart failure. Clin Res Cardiol 111:912–923
Authors/Task Force M, McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A and Group ESCSD. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131.
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Juni P, Pierard L, Prendergast BD, Sadaba JR, Tribouilloy C, Wojakowski W and Group EESD. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561-632
Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ, Investigators C (2018) Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 379:2307–2318
Stone GW, Abraham WT, Lindenfeld J, Kar S, Grayburn PA, Lim DS, Mishell JM, Whisenant B, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Asch FM, Mack MJ, Investigators C (2023) Five-year follow-up after transcatheter repair of secondary mitral regurgitation. N Engl J Med 388:2037–2048
Franzen O, van der Heyden J, Baldus S, Schluter M, Schillinger W, Butter C, Hoffmann R, Corti R, Pedrazzini G, Swaans MJ, Neuss M, Rudolph V, Surder D, Grunenfelder J, Eulenburg C, Reichenspurner H, Meinertz T, Auricchio A (2011) MitraClip(R) therapy in patients with end-stage systolic heart failure. Eur J Heart Fail 13:569–576
Adamo M, Barbanti M, Curello S, Fiorina C, Chiari E, Chizzola G, Capodanno D, Tamburino C, Metra M, Ettori F (2015) Effectiveness of MitraClip therapy in patients with refractory heart failure. J Interv Cardiol 28:61–68
Berardini A, Biagini E, Saia F, Stolfo D, Previtali M, Grigioni F, Pinamonti B, Crimi G, Salvi A, Ferrario M, De Luca A, Gazzoli F, Bacchi Reggiani ML, Raineri C, Sinagra G, Rapezzi C (2017) Percutaneous mitral valve repair: the last chance for symptoms improvement in advanced refractory chronic heart failure? Int J Cardiol 228:191–197
Adamo M, Godino C, Giannini C, Scotti A, Liga R, Curello S, Fiorina C, Chiari E, Chizzola G, Abbenante A, Visco E, Branca L, Fiorelli F, Agricola E, Stella S, Lombardi C, Colombo A, Petronio AS, Metra M, Ettori F (2019) Left ventricular reverse remodelling predicts long-term outcomes in patients with functional mitral regurgitation undergoing MitraClip therapy: results from a multicentre registry. Eur J Heart Fail 21:196–204
Geis NA, Puls M, Lubos E, Zuern CS, Franke J, Schueler R, von Bardeleben RS, Boekstegers P, Ouarrak T, Zahn R, Ince H, Senges J, Katus HA, Bekeredjian R (2018) Safety and efficacy of MitraClip therapy in patients with severely impaired left ventricular ejection fraction: results from the German Transcatheter Mitral Valve Interventions (TRAMI) registry. Eur J Heart Fail 20:598–608
Karam N, Stolz L, Orban M, Deseive S, Praz F, Kalbacher D, Westermann D, Braun D, Nabauer M, Neuss M, Butter C, Kassar M, Petrescu A, Pfister R, Iliadis C, Unterhuber M, Park SD, Thiele H, Baldus S, von Bardeleben RS, Blankenberg S, Massberg S, Windecker S, Lurz P, Hausleiter J (2021) Impact of right ventricular dysfunction on outcomes after transcatheter edge-to-edge repair for secondary mitral regurgitation. JACC Cardiovasc Imaging 14:768–778
Koell B, Orban M, Weimann J, Kassar M, Karam N, Neuss M, Petrescu A, Iliadis C, Unterhuber M, Adamo M, Giannini C, Melica B, Ludwig S, Massberg S, Praz F, Pfister R, Thiele H, Stephan von Bardeleben R, Baldus S, Butter C, Lurz P, Windecker S, Metra M, Petronio AS, Hausleiter J, Lubos E, Kalbacher D, Euro SMRI (2021) Outcomes stratified by adapted inclusion criteria after mitral edge-to-edge repair. J Am Coll Cardiol. 78:2408–2421
Chhatriwalla AK, Cohen DJ, Vemulapalli S, Vekstein A, Huded CP, Gallup D, Kosinski AS, Brothers L, Lindenfeld J, Stone GW, Sorajja P (2024) Transcatheter edge-to-edge repair in COAPT-ineligible patients with functional mitral regurgitation. J Am Coll Cardiol 83:488–499
Munafo AR, Scotti A, Estevez-Loureiro R, Adamo M, Hernandez AP, Peregrina EF, Gutierrez L, Taramasso M, Fam NP, Ho EC, Asgar A, Vitrella G, Raineri C, Chizzola G, Pezzola E, Le Ruz R, Montalto C, Oreglia JA, Fraccaro C, Giannini C, Fiorelli F, Rubbio AP, Ooms JF, Compagnone M, Marcelli C, Maffeo D, Bettari L, Furholz M, Arzamendi D, Guerin P, Tamburino C, Petronio AS, Grasso C, Agricola E, Van Mieghem NM, Tarantini G, Praz F, Pascual I, Potena L, Colombo A, Maisano F, Metra M, Margonato A, Crimi G, Saia F, Godino C (2023) 2-year outcomes of MitraClip as a bridge to heart transplantation: the international MitraBridge registry. Int J Cardiol 390:131139
Godino C, Munafo A, Scotti A, Estevez-Loureiro R, Portoles Hernandez A, Arzamendi D, Fernandez Peregrina E, Taramasso M, Fam NP, Ho EC, Asgar A, Vitrella G, Raineri C, Adamo M, Fiorina C, Montalto C, Fraccaro C, Giannini C, Fiorelli F, Popolo Rubbio A, Ooms JF, Compagnone M, Maffeo D, Bettari L, Furholz M, Tamburino C, Petronio AS, Grasso C, Agricola E, Van Mieghem NM, Tarantini G, Curello S, Praz F, Pascual I, Potena L, Colombo A, Maisano F, Metra M, Margonato A, Crimi G, Saia F (2020) MitraClip in secondary mitral regurgitation as a bridge to heart transplantation: 1-year outcomes from the International MitraBridge Registry. J Heart Lung Transplant 39:1353–1362
Bedogni F, Popolo Rubbio A, Grasso C, Adamo M, Denti P, Giordano A, Tusa M, Bianchi G, De Marco F, Bartorelli AL, Montorfano M, Godino C, Citro R, De Felice F, Mongiardo A, Monteforte I, Villa E, Giannini C, Crimi G, Tarantini G, Testa L, Tamburino C (2021) Italian Society of Interventional Cardiology (GIse) registry Of Transcatheter treatment of mitral valve regurgitaTiOn (GIOTTO): impact of valve disease aetiology and residual mitral regurgitation after MitraClip implantation. Eur J Heart Fail 23:1364–1376
Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge-Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska-Migaj E, McDonagh T, Seferovic P, Ruschitzka F (2018) Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 20:1505–1535
Mack MJ, Abraham WT, Lindenfeld J, Bolling SF, Feldman TE, Grayburn PA, Kapadia SR, McCarthy PM, Lim DS, Udelson JE, Zile MR, Gammie JS, Gillinov AM, Glower DD, Heimansohn DA, Suri RM, Ellis JT, Shu Y, Kar S, Weissman NJ, Stone GW (2018) Cardiovascular outcomes assessment of the MitraClip in patients with heart failure and secondary mitral regurgitation: design and rationale of the COAPT Trial. Am Heart J 205:1–11
Giustino G, Camaj A, Kapadia SR, Kar S, Abraham WT, Lindenfeld J, Lim DS, Grayburn PA, Cohen DJ, Redfors B, Zhou Z, Pocock SJ, Asch FM, Mack MJ, Stone GW (2022) Hospitalizations and mortality in patients with secondary mitral regurgitation and heart failure: the COAPT Trial. J Am Coll Cardiol 80:1857–1868
Adamo M, Fiorelli F, Melica B, D’Ortona R, Lupi L, Giannini C, Silva G, Fiorina C, Branca L, Chiari E, Chizzola G, Spontoni P, Espada Guerreiro C, Curello S, Petronio AS, Metra M (2021) COAPT-like profile predicts long-term outcomes in patients with secondary mitral regurgitation undergoing MitraClip implantation. JACC Cardiovasc Interv 14:15–25
Shuvy M, Maisano F (2024) Evolving indications for transcatheter mitral edge-to-edge repair. EuroIntervention 20:e230–e238
Pagnesi M, Calì F, Chiarito M, Stolfo D, Baldetti L, Lombardi CM, Tomasoni D, Loiacono F, Maccallini M, Villaschi A, Cocianni D, Perotto M, Voors AA, Pini D, Metra M, Adamo M (2024) HELP-HF Registry investigators. Prognostic role of mitralregurgitation in patients with advanced heart failure. Eur J Intern Med 122:102–108. https://doi.org/10.1016/j.ejim.2023.11.002
Shah N, Madhavan MV, Gray WA, Brener SJ, Ahmad Y, Lindenfeld J, Abraham WT, Grayburn PA, Kar S, Lim DS, Mishell JM, Whisenant BK, Zhang Z, Redfors B, Mack MJ, Stone GW (2022) Prediction of death or HF hospitalization in patients with severe FMR: the COAPT Risk Score. JACC Cardiovasc Interv 15:1893–1905
Kalbacher D, Schafer U, Vardeleben RS, Eggebrecht H, Sievert H, Nickenig G, Butter C, May AE, Bekeredjian R, Ouarrak T, Kuck KH, Plicht B, Zahn R, Baldus S, Ince H, Schillinger W, Boekstegers P, Senges J, Lubos E (2019) Long-term outcome, survival and predictors of mortality after MitraClip therapy: results from the German Transcatheter Mitral Valve Interventions (TRAMI) registry. Int J Cardiol 277:35–41
Adamo M, Pagnesi M, Popolo Rubbio A, Branca L, Grasso C, Denti P, Giordano A, Tusa M, De Marco F, Lupi L, Bartorelli AL, Godino C, Citro R, De Felice F, Mongiardo A, Monteforte I, Villa E, Giannini C, Testa L, Curello S, Tarantini G, Tamburino C, Bedogni F, Metra M (2022) Predictors of optimal procedural result after transcatheter edge-to-edge mitral valve repair in secondary mitral regurgitation. Catheter Cardiovasc Interv 99:1626–1635
Adamo M, Cani DS, Gavazzoni M, Taramasso M, Lupi L, Fiorelli F, Giannini C, Branca L, Zuber M, Curello S, Petronio AS, Maisano F, Metra M (2020) Impact of disproportionate secondary mitral regurgitation in patients undergoing edge-to-edge percutaneous mitral valve repair. EuroIntervention 16:413–420
Puls M, Lubos E, Boekstegers P, von Bardeleben RS, Ouarrak T, Butter C, Zuern CS, Bekeredjian R, Sievert H, Nickenig G, Eggebrecht H, Senges J, Schillinger W (2016) One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: results from the German Transcatheter Mitral Valve Interventions registry. Eur Heart J 37:703–712
Capodanno D, Adamo M, Barbanti M, Giannini C, Laudisa ML, Cannata S, Curello S, Imme S, Maffeo D, Bedogni F, Petronio AS, Ettori F, Tamburino C, Grasso C, Investigators G-I (2015) Predictors of clinical outcomes after edge-to-edge percutaneous mitral valve repair. Am Heart J 170:187–195
Adamo M, Gavazzoni M, Castiello A, Estevez-Loureiro R, Taramasso M, Lupi L, Branca L, Portoles A, Benito-Gonzalez T, Curello S, Maisano F, Metra M (2020) Prognostic impact of heart failure history in patients with secondary mitral regurgitation treated by MitraClip. Am J Cardiol 135:120–127
Lupi L, Italia L, Pagnesi M, Pancaldi E, Ancona F, Stella S, Pezzola E, Cimino G, Saccani N, Ingallina G, Margonato D, Inciardi RM, Lombardi CM, Tomasoni D, Agricola E, Metra M, Adamo M (2023) Prognostic value of right ventricular longitudinal strain in patients with secondary mitral regurgitation undergoing transcatheter edge-to-edge mitral valve repair. Eur Heart J Cardiovasc Imaging 24:1509–1517
Italia L, Adamo M, Lupi L, Scodro M, Curello S, Metra M (2021) Percutaneous edge-to-edge mitral valve repair: beyond the left heart. J Am Soc Echocardiogr 34:1038–1045
Adamo M, Inciardi RM, Tomasoni D, Dallapellegrina L, Estevez-Loureiro R, Stolfo D, Lupi L, Pancaldi E, Popolo Rubbio A, Giannini C, Benito-Gonzalez T, Fernandez-Vazquez F, Caneiro-Queija B, Godino C, Munafo A, Pascual I, Avanzas P, Frea S, Boretto P, Monivas Palomero V, Del Trigo M, Biagini E, Berardini A, Nombela-Franco L, Jimenez-Quevedo P, Lipsic E, Saia F, Petronio AS, Bedogni F, Sinagra G, Guazzi M, Voors A, Metra M (2022) Changes in right ventricular-to-pulmonary artery coupling after transcatheter edge-to-edge repair in secondary mitral regurgitation. JACC Cardiovasc Imaging 15:2038–2047
Higuchi S, Orban M, Stolz L, Karam N, Praz F, Kalbacher D, Ludwig S, Braun D, Nabauer M, Wild MG, Neuss M, Butter C, Kassar M, Petrescu A, Pfister R, Iliadis C, Unterhuber M, Park SD, Thiele H, Baldus S, von Bardeleben RS, Schofer N, Massberg S, Windecker S, Lurz P, Hausleiter J (2021) Impact of residual mitral regurgitation on survival after transcatheter edge-to-edge repair for secondary mitral regurgitation. JACC Cardiovasc Interv 14:1243–1253
Reichart D, Kalbacher D, Rubsamen N, Tigges E, Thomas C, Schirmer J, Reichenspurner H, Blankenberg S, Conradi L, Schafer U, Lubos E (2020) The impact of residual mitral regurgitation after MitraClip therapy in functional mitral regurgitation. Eur J Heart Fail 22:1840–1848
Kubo S, Yamamoto M, Saji M, Asami M, Enta Y, Nakashima M, Shirai S, Izumo M, Mizuno S, Watanabe Y, Amaki M, Kodama K, Yamaguchi J, Nakajima Y, Naganuma T, Bota H, Ohno Y, Yamawaki M, Ueno H, Mizutani K, Adachi Y, Otsuka T, Hayashida K, Investigators OC-M (2023) One-year outcomes and their relationship to residual mitral regurgitation after transcatheter edge-to-edge repair with MitraClip device: insights from the OCEAN-Mitral Registry. J Am Heart Assoc 12:e030747
von Bardeleben RS, Mahoney P, Morse MA, Price MJ, Denti P, Maisano F, Rogers JH, Rinaldi M, De Marco F, Rollefson W, Chehab B, Williams M, Leurent G, Asch FM, Rodriguez E (2023) 1-year outcomes with fourth-generation mitral valve transcatheter edge-to-edge repair from the EXPAND G4 study. JACC Cardiovasc Interv 16:2600–2610
Hausleiter J, Stocker TJ, Adamo M, Karam N, Swaans MJ, Praz F (2023) Mitral valve transcatheteredge-to-edge repair. EuroIntervention 18(12):957–976. https://doi.org/10.4244/EIJ-D-22-00725
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement. The GIOTTO Registry is sponsored by the Italian Society of Interventional Cardiology (GIse), which received a grant support for the study by Abbott Vascular, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.A., M.M., F.B., C.G., A.G., A.L.B., and C.T. received consultation and speaker fees from Abbott Vascular outside the submitted work. All other authors have nothing to disclose.
Additional information
Valeria Magni and Marianna Adamo are joint first authors.
Francesco Bedogni and Marco Metra are joint senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Magni, V., Adamo, M., Pezzola, E. et al. Impact of heart failure severity on the mortality benefit of mitral transcatheter edge-to-edge valve repair. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02490-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02490-7