Abstract
Introduction
Heart failure (HF) with mildly reduced and preserved ejection fraction (HFmrEF/HFpEF) is often accompanied by atrial dysfunction. It has been suggested that specific ectopic fat depots, such as epicardial adipose tissue (EAT), may directly influence the myocardial cells and, therefore, be involved in the pathophysiology of atrial mechanical dysfunction. In this study, we aimed to investigate the association between EAT and left atrial (LA) mechanical dysfunction.
Methods and results
In total, 82 patients with symptomatic HF and left ventricular ejection fraction > 40% were prospectively enrolled. All patients underwent CMR while in sinus rhythm. LA mechanical dysfunction was defined as the presence of LA end-systolic volume index > 52 mL/m2 and LA reservoir strain < 23%. EAT volume was indexed for body surface area. Mean age was 69 ± 10 years, 42 (51%) were women and mean body mass index (BMI) was 29 ± 6 kg/m2. Mean LVEF was 55 ± 9% and 34 (41%) patients had LA mechanical dysfunction. In patients with LA mechanical dysfunction, the EAT volume was significantly higher than in patients without LA mechanical dysfunction (90 vs 105 mL/m2, p = 0.02) while BMI was similar. In multivariable logistic regression analyses, increased EAT remained significantly associated with LA mechanical dysfunction (OR 1.31, 95% CI 1.03–1.66, p = 0.03).
Conclusion
Increased EAT was associated with LA mechanical dysfunction in patients with HFmrEF and HFpEF. Further research is needed to elucidate the exact mechanisms that underlie this association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence rate of heart failure (HF) with mildly reduced or preserved ejection fraction (HFmrEF/HFpEF) is increasing to epidemic proportions, and this type of HF is significantly contributing to global morbidity and mortality [1]. Evidence suggests that HFmrEF and HFpEF have multiple overlapping pathophysiological mechanisms that all contribute to the development of the disease, including risk factors such as ageing, hypertension, diabetes mellitus and obesity [2]. Furthermore, in the course of the disease, enlargement of the left atrium (LA) and loss of its functions (as reservoir, conduit and ejection chamber) are hallmark findings [3, 4].
Obesity seems one of the most important comorbidities in the development of HFmrEF/HFpEF [5]. It also seems an important contributor to atrial dysfunction in these patients, possibly through activation of inflammatory and neurohumoral systems, as well as cellular-level modifications [6]. In this regard, especially epicardial adipose tissue (EAT) may be directly involved in the pathophysiology of atrial dysfunction in patients with HFmrEF/HFpEF [7]. EAT is the fat depot located in direct contact with the myocardium, and by this direct connection causing myocardial dysfunction [8, 9]. However, data on the relation between EAT and cardiac magnetic resonance imaging (CMR)-derived atrial dysfunction is scarce. We therefore investigated the association between EAT and LA mechanical dysfunction in patients with HFmrEF/HFpEF, using CMR.
Methods
Study population
All 125 patients included in the present study had symptomatic HF with New York Heart Association class II or III, left ventricular ejection fraction (LVEF) > 40% and N-terminal pro-hormone of brain natriuretic peptide (NT-proBNP) level > 125 ng/L. In addition, all patients needed to have echocardiographic evidence of left ventricular (LV) diastolic dysfunction, LA dilatation and/or LV hypertrophy [10].
The majority of the patients in this study (105, 84%) were enrolled in the Ventricular Tachyarrhythmia Detection by Implantable Loop Recording in Patients with Heart Failure and Preserved Ejection Fraction (VIP-HF) study and they provided written informed consent [11]. The remaining 20 patients were screened for the VIP-HF study, but did not meet all the inclusion and exclusion criteria, or refused the implantation of a loop recorder. The use of non-VIP-HF HF patients was approved by the local ethics committee. The investigation conforms with the principles outlined in the Declaration of Helsinki.
All patients underwent a standard diagnostic work-up for HF patients with LVEF > 40% at our centre, including medical history, physical examination, laboratory testing, echocardiography and CMR. For the present study, only patients who were in sinus rhythm during CMR assessment were included and patients with atrial fibrillation (AF) during CMR were excluded (n = 42). Patients were also excluded from the present analysis if no sufficient CMR images were available to assess LA parameters (n = 1).
Cardiac magnetic resonance imaging
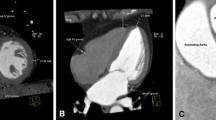
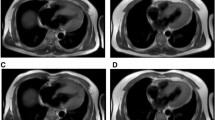
CMR was performed using a standard protocol for the acquisition of cardiac volume, function and mass. The used protocol is previously described [12]. In brief, CMR acquisitions were performed on a 1.5-T scanner (Siemens, Erlangen, Germany & Philips, Amsterdam, The Netherlands). Typical sequence parameters for the scanners were as follows: for Siemens: TE 1.08 ms; TR 700 ms; flip angle 40°; voxel size 2.3 × 2.3 × 6.0 mm; and for Philips: TE 0.93 ms; TR 2.1 ms; flip angle 25°; voxel size 1.6 × 1.8 × 8.0 mm. ECG-triggered cine loop images were obtained during breath hold at end-expiration using a retrospectively gated cine steady-state, free-precession sequence. Approximately 15 short-axis slices from base to apex were obtained, including the atria.
Cine loop images were analysed off-line by experienced observers using dedicated software (QMass 7.6 and 8.1, QStrain 2.0, Medis, Leiden, The Netherlands) [12]. Endo- and epicardial borders of the left and right ventricles were manually delineated on the end-diastolic and end-systolic phases on the short-axis stacks. End-diastolic volumes and end-systolic volumes were automatically calculated by the summation of slices multiplied by slice thickness method. Volumetric measurements were indexed for body surface area, according to the Mosteller formula [13]. Strain was measured as the total deformation of the myocardium from its baseline length to its maximum length, and is expressed as a percentage. Longitudinal LV strain and RV strain were measured on cine images. Using the long-axis slices, left and right atrial volumes were measured by tracing the area and length of both atria in end-systole and end-diastole. Atrial volume was approximated using the biplane area-length method. Subsequently, LA reservoir, conduit and contractile strain were assessed using cine long-axis two- and four-chamber acquisitions.
Left atrial function
Left atrial mechanical dysfunction was defined as the combination of abnormal LA end-systolic volume indexed for body surface area and abnormal LA reservoir strain, both acquired from CMR. LA end-systolic volume index and LA reservoir strain were abnormal according to previously published cutoff values (i.e. > 52 mL/m2 for LA end-systolic volume index; and < 23% for LA reservoir strain) [14,15,16]. If none or only one of these measures was abnormal, LA mechanical dysfunction was not present.
Epicardial adipose tissue
Epicardial adipose tissue was defined as the adipose tissue situated between the outer wall of the myocardium and the visceral layer of the pericardium [17, 18]. Epicardial adipose tissue was measured using the cine short-axis for contouring and the cine long-axis stacks for referencing. While delineating the short axis, cine long-axis images were available for reference to ensure accurate differentiation of the selected area. Short-axis T1 mapping at three levels (base, mid and apex) was used to confirm adipose tissue, as described previously [12]. After confirmation, the EAT part on the cine short-axis slices was extrapolated beyond the part that T1 maps were available. Epicardial adipose tissue volumes were calculated by summation of EAT volume of each slice using the modified Simpson’s rule and indexed for body surface area according to the Mosteller formula [13, 19].
Statistical analysis
Continuous data are presented as mean ± standard deviation (SD) or median (interquartile range, IQR) depending on the distribution, while categorical data are presented as numbers (percentage). Patient characteristics between the patients with and without LA mechanical dysfunction were compared using an independent T-test or Mann–Whitney U test depending on their distribution, or a chi-square test or Fisher’s exact test for categorical variables.
Logistic regression analysis was used to assess the association between EAT and LA mechanical dysfunction. First, the association between EAT and LA mechanical dysfunction was univariably tested (model 1). Potential confounders/interacting covariates were selected based on the literature [20,21,22]. Multivariable regression analyses were used to adjust EAT for age, sex and body mass index (BMI) (model 2), and for adjustment for age, sex, BMI, LVEF, diabetes mellitus, history of AF, and myocardial infarction (model 3). Tests were performed with SPSS version 28 (IMB, Armonk, NY). A p-value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 82 patients were included. The mean age was 69 ± 10 years, 42 (51%) were women, mean BMI was 29 ± 6 kg/m2 and 26 (32%) had a history of AF. In the selected patient group, one remaining patient had a history of ablation. Mean LVEF was 55 ± 9%, mean LA end-systolic volume index was 54 ± 19 mL/m2 and mean LA reservoir strain was 18 ± 9%. The characteristics of the study population are shown in Table 1.
Left atrial mechanical dysfunction
In 38 patients (46%), LA end-systolic volume was > 52 mL/m2, and in 62 patients (76%), LA reservoir strain was < 23%. In total, 34 patients (41%) had LA mechanical dysfunction according to our definition. Differences in characteristics between patients with and without LA mechanical dysfunction are also depicted in Table 1. Overall, most general characteristics were comparable between the two groups, including having a history of AF. Hereby, it should be noted that patients with AF at baseline were excluded from this analysis.
As expected, patients with LA mechanical dysfunction had also larger LA volumes on echocardiography, and lower LA conduit and contractile strain (Fig. 1). Patients with LA mechanical dysfunction had higher E/e' and larger RV and RA volumes.
Association between EAT and LA mechanical dysfunction
Patients with LA mechanical dysfunction had a significantly higher amount of total EAT than patients without LA mechanical dysfunction (90 vs 105 mL/m2, p = 0.02), while BMI was similar between both groups (Fig. 2). The logistic regression analysis is shown in Table 2. In the unadjusted model (model 1), total EAT was significantly associated with LA mechanical dysfunction (OR 1.25, 95% CI 1.03–1.53, p = 0.02). After adjusting for either age, sex or BMI (model 2) as well as for age, sex, BMI, history of AF, diabetes mellitus, myocardial infarction and LVEF (model 3), the association between EAT and LA mechanical dysfunction remained significant. The location-specific analysis and analysis in only HFpEF patients have been added to the supplement as Supplementary Tables 1 and 2 respectively.
Discussion
In the present study, we showed that there is an association between EAT and LA mechanical dysfunction in patients with HFmrEF/HFpEF who were in sinus rhythm. Interestingly, this association was independent of BMI, potentially indicating that EAT plays a role in the pathophysiology of LA mechanical dysfunction in HFpEF irrespective of overall obesity.
Left atrial mechanical dysfunction is indicative of adverse LA remodelling, an important substrate for the development of AF [23]. The association between increased EAT and higher prevalence of AF has previously been shown [24]. However, only limited research has been conducted on the direct association between EAT and LA mechanical dysfunction. Evin et al. demonstrated in a small cohort of 19 controls and 20 non-HF patients with obesity and diabetes mellitus type 2 that LA strain measured by CMR was associated with EAT [25]. Additionally, Jin et al. also found an association between EAT thickness — measured by echocardiography — and LA and LV function in patients with HFmrEF/HFpEF [26]. However, only a modest correlation exists between EAT measured by echocardiography in comparison with CMR [27]. Our study presents the first data regarding the association between EAT and LA mechanical dysfunction in patients with HFmrEF/HFpEF, both measured by CMR.
Several mechanisms underlying the association between increased EAT and LA mechanical dysfunction have been postulated, such as increased inflammation, infiltration and accumulation of fibrosis as well as mechanical effects. Most mechanisms are predicated upon the observation that EAT and myocardial cells are directly connected without the presence of an intervening fascia, but the exact mechanisms remain poorly understood. Among others, the different mechanisms are elucidated in recent published reviews by our research group and Iacobellis [9, 28]. These reviews highlight that the direct interaction between EAT and the underlying myocardium enables EAT to communicate with the underlying myocardium. Therefore, it is possible that EAT may release harmful mediators to the myocardial cells, which infiltrate the ultrastructure of the myocardium and potentially lead to upregulation of inflammation [28]. The inflammatory characteristics of EAT itself may also lead to paracrine effects which cause fatty infiltration or accumulation of fibrosis [29, 30]. Next to these effects by mediators, there is evidence that EAT can induce mechanical effects by compressing the cardiac myocardium, leading to diastolic dysfunction and increased intracardiac pressures [31]. It is likely that a combination of factors reinforces each other, amplifying the influence of EAT on the heart. Considering these hypotheses, it would be reasonable to assume that EAT primarily exerts at local level and that atrial EAT plays a role in the onset and progression of LA remodelling, and therefore, in LA mechanical dysfunction. The present study did not show a significant association between atrial EAT and LA mechanical dysfunction. The amount of atrial EAT in our analysis included both left and right atrial EAT. Unfortunately, no data was available on LA EAT separately, which could be one of the possible explanations of the non-significant association between atrial EAT and LA mechanical dysfunction. Other explanations can be found within the mechanisms underlying the association between EAT and myocardial dysfunction, where the exact pathophysiological mechanisms of EAT remain unclear. Hypothetically, EAT surrounding the entire heart may also have more effect on LA mechanical dysfunction through hemodynamic mechanical effects than local atrial EAT. The lack of significance in the association between atrial EAT and LA mechanical dysfunction could also be attributed to varying contributions of the different hypotheses proposed.
Building upon LA remodelling, there has been a growing interest in atrial cardiomyopathy, whose exact definition is yet to be determined [3]. Kreimer and Gotzmann have proposed a model categorizing atrial cardiomyopathy into three components, i.e. mechanical dysfunction, atrial fibrosis and electrical dysfunction [32]. In this study, our focus was on CMR-derived LA mechanical dysfunction, as we encountered limitations in assessing LA fibrosis and electrical dysfunction. Specifically, our imaging sequences were not suitable for evaluating LA fibrosis, and we did not conduct voltage maps, which are required for assessing LA fibrosis. Furthermore, our standard 12-lead ECGs were inadequate for measuring electrical dysfunction, as they lacked the necessary digitally stored data for post-processing with specific software. We defined LA mechanical dysfunction as the combination of LA end-systolic volume index and LA reservoir strain. LA end-systolic volume index is a widely used parameter and is one of the most important determinants of atrial remodelling [33]. Additionally, LA strain provides insights into the functionality of the LA [34]. Since the presence of AF lasting longer than 48 h leads to the possibility of LA stunning and subsequently lower LA strain values, only patients in sinus rhythm were included [14]. The chosen cutoff values of LA end-systolic volume index and LA reservoir strain are based on previously published values [14,15,16]. However, due to the relative novel modality, normal ranges of both parameters in CMR still need to be confirmed [35,36,37].
In future research, it would be intriguing to combine all three described components of atrial cardiomyopathy to further explore this definition and evaluate the association between EAT and atrial function.
Limitations
First, the relative modest sample size limits the ability to adjust for multiple confounders. The relative modest sample size also limits the statistical power of the cohort, especially when focusing solely on HFpEF patients. Second, patients with implantable cardiac devices were excluded from the study, which potentially limits the generalizability of the findings. Third, scanners from two different vendors were used for the CMR acquisitions. Albeit the vast majority of scans were performed on a single Siemens scanner (n = 74, 90%), we anticipate minimal impact on the results, especially regarding the measurements of the LA size and function and the volume of EAT. Fourth, the cross-sectional nature of the study limits the investigation of a causal relation between increased EAT and LA mechanical dysfunction.
Conclusion
Increased EAT was associated with LA mechanical dysfunction in patients with HF with mildly reduced or preserved ejection fraction. Further research is needed to elucidate the exact mechanisms that underlie the association between EAT and LA mechanical dysfunction.
References
Savarese G, Lund LH (2017) Global public health burden of heart failure. Card Fail Rev 3(1):7–11
Kotecha D, Lam CSP, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M (2016) Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol 68(20):2217–2228
Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA et al (2016) EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterisation, and clinical implication. J Arrhythm 32(4):247–278
Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JPM et al (2015) Electrophysiological, electroanatomical, and structural remodeling of the atria as consequences of sustained obesity. J Am Coll Cardiol 66(1):1–11
Abed HS, Samuel CS, Lau DH, Kelly DJ, Royce SG, Alasady M et al (2013) Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm 10(1):90–100
Middeldorp ME, Kamsani SH, Sanders P (2023) Obesity and atrial fibrillation: prevalence, pathogenesis, and prognosis. Prog Cardiovasc Dis 78:34–42
Packer M (2018) The epicardial adipose inflammatory triad: coronary atherosclerosis, atrial fibrillation, and heart failure with a preserved ejection fraction. Eur J of Heart Fail 20(11):1567–1569
Iacobellis G, Corradi D, Sharma AM (2005) Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2(10):536–543
Iacobellis G (2022) Epicardial adipose tissue in contemporary cardiology. Nat Rev 19:593–606
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS et al (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 37(27):2129–2200
van Veldhuisen DJ, van Woerden G, Gorter TM, van Empel VPM, Manintveld OC, Tieleman RG et al (2020) Ventricular tachyarrhythmia detection by implantable loop recording in patients with heart failure and preserved ejection fraction: the VIP-HF study. Eur J Heart Fail 22(10):1923–1929
van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M (2018) Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail 20(11):1559–1566
Mosteller RD (1987) Simplified calculation of body-surface area. N Engl J Med 317(17):1098
Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, Donal E et al (2022) Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 23(2):E34-61
Petersen SE, Aung N, Sanghvi MM, Zemrak F, Fung K, Paiva JM et al (2017) Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson 19(1)
Nielsen AB, Skaarup KG, Hauser R, Johansen ND, Lassen MCH, Jensen GB et al (2022) Normal values and reference ranges for left atrial strain by speckle-tracking echocardiography: the Copenhagen City Heart Study. Eur Heart J Cardiovasc Imaging 23(1):42–51
Doesch C, Streitner F, Bellm S, Suselbeck T, Haghi D, Heggemann F et al (2013) Epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure due to dilated cardiomyopathy. Obesity 21(3)
Iacobellis G (2009) Epicardial and pericardial fat: close, but very different. Obesity 17(4):625
Flüchter S, Haghi D, Dinter D, Heberlein W, Kühl HP, Neff W et al (2007) Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity 15(4):870–878
Goette A, Lendeckel U (2021) Atrial cardiomyopathy: pathophysiology and clinical consequences. Cells 10(2605)
Andrade J, Khairy P, Dobrev D, Nattel S (2014) The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 114(9):1453–1468
Suthahar N, Meijers WC, Silljé HHW, de Boer RA (2017) From inflammation to fibrosis—molecular and cellular mechanisms of myocardial tissue remodelling and perspectives on differential treatment opportunities. Curr Heart Fail Rep 14(4):235–250
Delgado V, Di BL, Leung M, Romero J, Tops LF, Casadei B et al (2017) Structure and function of the left atrium and left atrial appendage AF and stroke implications. J Am Coll Cardiol 70(25):3157–3172
Gaeta M, Bandera F, Tassinari F, Capasso L, Cargnelutti M, Pelissero G et al (2017) Is epicardial fat depot associated with atrial fibrillation? A systematic review and meta-analysis. Europace 19(5):747–752
Evin M, Broadhouse KM, Callaghan FM, McGrath RT, Glastras S, Kozor R et al (2016) Impact of obesity and epicardial fat on early left atrial dysfunction assessed by cardiac MRI strain analysis. Cardiovasc Diabetol 15(1)
Jin X, Hung CL, Tay WT, Soon D, Sim D, Sung KT et al (2022) Epicardial adipose tissue related to left atrial and ventricular function in heart failure with preserved versus reduced and mildly reduced ejection fraction. Eur J Heart Fail 24(8):1346–1356
van Woerden G, van Veldhuisen DJ, Gorter TM, Ophuis B, Saucedo-Orozco H, van Empel VPM et al (2022) The value of echocardiographic measurement of epicardial adipose tissue in heart failure patients. ESC Heart Fail 9(2):953–957
van Woerden G, van Veldhuisen DJ, Westenbrink BD, de Boer RA, Rienstra M, Gorter TM (2022) Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: mechanisms, management and modern perspectives. Eur J Heart Fail 24(12):2238–2250
Nalliah CJ, Bell JR, Raaijmakers AJA, Waddell HM, Wells SP, Bernasochi GB et al (2020) Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol 76(10):1197–1211
Packer M (2018) Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 71(20):2360–2372
Gorter TM, van Woerden G, Rienstra M, Dickinson MG, Hummel YM, Voors AA et al (2020) Epicardial adipose tissue and invasive hemodynamics in heart failure with preserved ejection fraction. JACC Heart Fail 8(8):667–676
Kreimer F, Gotzmann M (2022) Left atrial cardiomyopathy – a challenging diagnosis. Front Cardiovasc Med 9
To ACY, Flamm SD, Marwick TH, Klein AL (2011) Clinical utility of multimodality LA imaging assessment of size, function, and structure. JACC Cardiovasc Imaging 4(7):788–798
Roşca M, Lancellotti P, Popescu BA, Piérard LA (2011) Left atrial function: pathophysiology, echocardiographic assessment, and clinical applications. Heart 97(23):1982–1989
Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S et al (2010) Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 3(3):231–239
Benjamin MM, Munir MS, Shah P, Kinno M, Rabbat M, Sanagala T et al (2022) Comparison of left atrial strain by feature-tracking cardiac magnetic resonance with speckle-tracking transthoracic echocardiography. Int J of Cardiovasc Imaging 38(6):1383–1389
Pathan F, D’Elia N, Nolan MT, Marwick TH, Negishi K (2017) Normal ranges of left atrial strain by speckle-tracking echocardiography: a systematic review and meta-analysis. J Am Soc Echocardiogr 30(1):59-70.e8
Funding
The VIP-HF study was supported by an unrestricted grant from Abbott-Netherlands to the University Medical Center Groningen. Abbott-Netherlands was neither involved in the conduction of the study, nor in the writing of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lobeek, M., Gorter, T.M., Westenbrink, B.D. et al. Increased epicardial adipose tissue is associated with left atrial mechanical dysfunction in patients with heart failure with mildly reduced and preserved ejection fraction. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02466-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02466-7