Abstract
Background and aims
Candidate selection for lung transplantation (LuTx) is pivotal to ensure individual patient benefit as well as optimal donor organ allocation. The impact of coronary artery disease (CAD) on post-transplant outcomes remains controversial. We provide comprehensive data on the relevance of CAD for short- and long-term outcomes following LuTx and identify risk factors for mortality.
Methods
We retrospectively analyzed all adult patients (≥ 18 years) undergoing primary and isolated LuTx between January 2000 and August 2021 at the LMU University Hospital transplant center. Using 1:1 propensity score matching, 98 corresponding pairs of LuTx patients with and without relevant CAD were identified.
Results
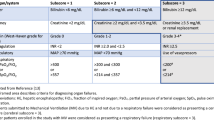
Among 1,003 patients having undergone LuTx, 104 (10.4%) had relevant CAD at baseline. There were no significant differences in in-hospital mortality (8.2% vs. 8.2%, p > 0.999) as well as overall survival (HR 0.90, 95%CI [0.61, 1.32], p = 0.800) between matched CAD and non-CAD patients. Similarly, cardiovascular events such as myocardial infarction (7.1% CAD vs. 2.0% non-CAD, p = 0.170), revascularization by percutaneous coronary intervention (5.1% vs. 1.0%, p = 0.212), and stroke (2.0% vs. 6.1%, p = 0.279), did not differ statistically between both matched groups. 7.1% in the CAD group and 2.0% in the non-CAD group (p = 0.078) died from cardiovascular causes. Cox regression analysis identified age at transplantation (HR 1.02, 95%CI [1.01, 1.04], p < 0.001), elevated bilirubin (HR 1.33, 95%CI [1.15, 1.54], p < 0.001), obstructive lung disease (HR 1.43, 95%CI [1.01, 2.02], p = 0.041), decreased forced vital capacity (HR 0.99, 95%CI [0.99, 1.00], p = 0.042), necessity of reoperation (HR 3.51, 95%CI [2.97, 4.14], p < 0.001) and early transplantation time (HR 0.97, 95%CI [0.95, 0.99], p = 0.001) as risk factors for all-cause mortality, but not relevant CAD (HR 0.96, 95%CI [0.71, 1.29], p = 0.788). Double lung transplant was associated with lower all-cause mortality (HR 0.65, 95%CI [0.52, 0.80], p < 0.001), but higher in-hospital mortality (OR 2.04, 95%CI [1.04, 4.01], p = 0.039).
Conclusion
In this cohort, relevant CAD was not associated with worse outcomes and should therefore not be considered a contraindication for LuTx. Nonetheless, cardiovascular events in CAD patients highlight the necessity of control of cardiovascular risk factors and a structured cardiac follow-up.
Similar content being viewed by others
Introduction
Advanced lung disease is associated with severely impaired quality of life and dramatically reduced life expectancy [1,2,3]. Though well established, lung transplantation as the ultimate therapeutic option is highly limited by the scarcity of donor organs [4, 5]. Therefore, thorough evaluation and candidate selection is pivotal to ensure individual benefit from transplantation as well as optimal resource allocation. In LuTx candidates, cardiovascular co-morbidities are common and a relevant proportion of patients with advanced lung disease is known to suffer from occult coronary artery disease (CAD) [6,7,8,9,10]. End-stage pulmonary fibrosis (PF) as well as chronic obstructive pulmonary disease (COPD), for example, are associated with an increased prevalence of CAD – reported up to 35% [10,11,12,13]. This association is still insufficiently understood and only partially explained by shared risk factors such as tobacco use [14]. Additionally, relevant CAD has become more prevalent in patients undergoing LuTx due to the increasing age of recipients. Consequently, coronary angiography is routinely performed in candidates older than 40 years or those with elevated cardiovascular risk in the majority of transplantation centers. However, the interpretation of the results, particularly regarding their clinical significance for LuTx outcomes, remains challenging.
While CAD has historically been considered a relative contraindication for LuTx [15], small retrospective single-center studies suggested that the presence of CAD prior to LuTx might not significantly increase the rate of peri- and postoperative cardiovascular events or mortality and that outcomes are acceptable in selected patients undergoing coronary revascularization prior to or concomitant with LuTx [16, 17]. However, published data regarding obstructive CAD with coronary artery stenosis > 50% or necessity for revascularization and long-term outcomes are sparse and partially inconclusive. Thus, while some studies found similar overall survival in patients with obstructive CAD, others have demonstrated impaired long-term survival [18,19,20,21,22]. Albeit most studies were limited by small sample sizes and short follow-up periods. Furthermore, contradicting data concerning cardiovascular events and death following LuTx further complicates cardiovascular candidate evaluation. For instance, studies investigating LuTx and coronary artery bypass grafting (CABG) showed that CABG prior to LuTx might be associated with increased mortality [23], while simultaneous CABG on the other hand doesn’t seem to affect survival [24]. Currently, the International Society for Heart and Lung Transplantation (ISHLT), as stated in its 2021 consensus document for the selection of LuTx candidates, does not consider CAD as an absolute contraindication but rather a risk factor for impaired outcomes and a potential marker for an unfavorable phenotype [25].
Overall, the available evidence is insufficient to evaluate the relevance of relevant CAD in the context of LuTx. Therefore, our study aimed to better understand the role of relevant CAD in LuTx by focusing on short- and long-term survival, cardiovascular events and death following LuTx in patients with and without relevant CAD as well as independent risk factors for mortality in this patient population.
Methods
Study design and patient selection
We retrospectively analyzed all adult patients (≥ 18 years) undergoing primary and isolated LuTx between January 2000 and August 2021 at the LMU University Hospital transplant center. Patients receiving multi-organ transplantation or re-transplantation were excluded. Patient data were extracted from the central clinical database, with subsequent strict data anonymization. Validity and integrity of the clinical research dataset was controlled by two senior physicians and by our statistical team. Standardized definitions for comorbidities and a data dictionary were used. Statistical analysis was prespecified and the statistical analysis plan was written before the data was received by the statistical analysis team. This is the primary analysis of this dataset, which was exclusively collected to investigate the role of relevant CAD in LuTx patients.
Selection and management of LuTx patients
Patient selection was performed following interdisciplinary discussion in the transplantation conference and in accordance with contemporary ISHLT selection guidelines [15, 25]. As per institution guidelines, CAD was assessed in all potential LuTx candidates older than 40 years and in patients with either angina pectoris or a profound cardiovascular risk (arterial hypertension and dyslipidaemia) by routine invasive coronary angiography. In patients who had already undergone percutaneous coronary intervention (PCI) before evaluation for a transplantation, coronary angiography was repeated at the time of listing. Patients were not excluded from LuTx because of CAD if (1) the coronary system was correctable, if (2) coronary perfusion in non-invasive stress testing was sufficient, and if (3) left ventricular ejection fraction (LVEF) was not severely impaired (LVEF > 35%). If patients were diagnosed with CAD requiring revascularization during pretransplant cardiovascular evaluation, revascularization was performed according to the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) guidelines after interdisciplinary determination of the best individual revascularization strategy. Depending on the urgency of LuTx, listing was postponed between 3 and 6 months after stenting because of dual antiplatelet therapy. At the time of listing, P2Y12-inhibitors were stopped with continued single antiplatelet therapy based on aspirin. A center-specific structured surveillance and follow-up including outpatient and inpatient visits was conducted and follow-up schedules were adapted to the requirements of each patient with at least yearly posttransplant follow-ups in stable patients. Standard immunosuppression regimen consisted of a triple combination with steroids, mycophenolate mofetil and tacrolimus.
Definition of relevant CAD
Relevant CAD was defined as (1) presence of epicardial coronary artery stenosis > 50% seen in the coronary angiography, (2) prior necessity of revascularization by PCI or CABG, or (3) prior myocardial infarction (MI). CAD grading in 1–3 vessel disease was correspondingly defined by presence of either coronary artery stenosis > 50% or prior revascularization in the left anterior descending (LAD), left circumflex artery (LCX) or right coronary artery (RCA). Lesions of the left main artery (LM) were thereby treated as combined lesions of LAD and LCX.
Clinical endpoints
The primary outcome variable was post-transplant survival, ascertained from the date of transplantation until patient death, date of last follow-up as recorded in the electronic health record or the end of study period. Patients were censored if alive at study termination. Secondary endpoints were obtained from a prospectively recorded data base and included in-hospital mortality, post-transplant cardiovascular (MI, necessity of coronary angiography, PCI or CABG, stroke, thrombosis or pulmonary embolism, atrial fibrillation, and cardiac arrest), non-cardiovascular events (dialysis, reoperation) and cause of death (bleeding, chronic allograft dysfunction, cardiovascular, infection/sepsis, malignancy, multi-organ failure and mors in tabula).
Risk factor analysis
Based on clinical reasoning and prior evidence from the literature [25,26,27,28,29,30], we considered the following set of risk factors for in-hospital mortality and all-cause mortality in our study: age at transplantation, sex, hypertension, diabetes, smoking, body mass index (BMI), creatinine, bilirubin, restrictive lung disease, obstructive lung disease, forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), CAD, LVEF, tricuspid annular plane systolic excursion (TAPSE), tricuspid valve regurgitation (TR), mean pulmonary artery pressure (mPAP), pulmonary capillary wedge pressure (PCWP), double lung transplantation, reoperation, bridge to transplant using extracorporeal membrane oxygenation (ECMO) and time since first transplantation, i.e. time difference between the transplantation time of the individual patient and the first transplantation on January 5th, 2000, to take transplant era into account. We performed risk factor analysis using Cox regression and logistic regression for all-cause mortality and in-hospital mortality, respectively. In order to avoid overfitting, we did not perform variable selection on this set of pre-specified risk factors but included all variables in the multiple logistic regression model.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics boards. Ethical approval for this study was obtained from the university hospital’s institutional ethic committee (approval number Az 19–630).
Statistical analysis
Statistical analysis was performed using R® (version 4.2.1, The R Foundation, Vienna, Austria). Kolmogorov–Smirnov-test, D’Agostino–Pearson-omnibus-test, q-q-plots and histograms were used to test the normality of data distribution. Normally distributed continuous variables were reported as a mean with standard deviation and non-normally distributed continuous variables as a median with interquartile ranges (25th and 75th percentile). To compare two groups, paired and unpaired t-test for normally distributed continuous variables and paired and unpaired Mann–Whitney-U test for non-normally distributed continuous variables were used. Categorical variables were reported as absolute numbers and percentages. To compare unpaired groups, chi-squared test and for matched paired groups, McNemar’s test was used. All tests were 2-tailed, and p-values < 0.05 were considered significant. Survival probabilities were calculated using the Kaplan–Meier method and comparisons were made by using stratified log-rank tests. For propensity score matching, the R package “MatchIt” (version 4.5.0) was utilized with a 1:1 nearest neighbor algorithm, no replacement, logistic link distance measure and a cutoff threshold of 0.2 standardized mean differences was applied. The propensity score was estimated by logistic regression. Based on previous published literature as well as clinical rationale the following baseline parameters were used for matching: age at time of transplantation, sex, BMI, diabetes, hypertension, smoking, bridge to transplant using ECMO, double lung transplantation, FEV1, FVC, CI, mPAP and PCWP [25,26,27,28,29,30].
Results
Study population
During the study period, a total of 1,051 LuTx patients were evaluated. After exclusion of pediatric patients (12), patients undergoing re-transplantation (27) or multi-organ transplantation (5) and patients with incomplete data or missing follow-up (4), 1,003 patients were included in the analysis (Fig. 1). In total, 98 LuTx patients with relevant CAD could be matched with 98 LuTx patients without CAD, achieving a standard difference of mean below 0.2 for all matching parameters (Supp. Figure 1). The median follow-up was 3.24 [1.30, 4.82] years. In the overall cohort, the median age was 54.7 years [45.8, 60.5] with 547 (54.5%) being male. Restrictive lung disease was the most frequent reason for LuTx (502, 50.0%), followed by obstructive lung disease (289, 28.8%), cystic fibrosis (139, 13.9%), and pulmonary vascular disease (40, 4.0%). The prevalence of CAD including mild CAD and coronary sclerosis was 23%. Relevant CAD was present in 104 (10.4%) patients, with the majority suffering from 1-vessel CAD (64, 6.4%), followed by 2-vessel (23, 2.3%) and 3-vessel disease (17, 1.7%). The most frequently affected coronary artery was the LAD (51, 5.1%), followed by RCA (28, 2.8%), LCX (24, 2.4%) and LM (2, 0.2%). 19 patients (1.9%) had suffered prior MI and revascularization had been performed in 60 patients (6.0%) via PCI while 4 patients (0.4%) had undergone CABG. Detailed baseline characteristics before and after matching can be found in Table 1 and 2, respectively.
Flow diagram depicting patient selection. 1,051 patients receiving lung transplantation between 01/2000 and 08/2021 were screened, 48 patients met exclusion criteria and were removed from the analysis. A total of 1,003 patients was included and divided in patients with relevant coronary artery disease (n = 104) and patients without relevant coronary artery disease (n = 899). Using 1:1 propensity score matching 98 corresponding pairs of LuTx patients with and without relevant CAD were identified. CAD = coronary artery disease, PCI = Percutaneous coronary intervention, CABG = coronary artery bypass graft
Differences in baseline characteristics between patients without and with relevant CAD
Clinical and practical significant differences between unmatched patients with and without relevant CAD were observed concerning age at transplantation [y] (60.3 [56.7, 63.2] vs. 54.0 [44.5, 59.9], p < 0.001), sex [male] (74.0% vs. 52.3%, p < 0.001) and BMI [kg/m2] (24.1 [21.3, 27.4] vs. 22.2 [19.4, 25.6], p < 0.001) as well as the abundance of cardiovascular risk factors including hypertension (67.3% vs. 32.1%, p < 0.001) and history of tobacco use (67.3% vs. 40.9%, p < 0.001). Furthermore, patients with relevant CAD underwent transplants more often for restrictive lung disease (71.2% vs. 47.6%, p < 0.001), had lower CI [L/m2] (2.95 [2.50, 3.40] vs. 3.10 [2.68, 3.70], p = 0.012), more often reduced LVEF (11.6% vs. 5.4%, p = 0.027), a higher rate of carotid plaques (20.2% vs. 8.5%, p < 0.001) and a higher FEV1 [% of reference] (44.00 [23.00, 56.25] vs. 30.00 [20.55, 46.00], p < 0.001) (Tab. 1). In the matched cohort, patients with relevant CAD significantly more often had coronary sclerosis (100% vs. 27.6%, p < 0.001), suffered prior MI (17.3% vs. 0.0%, p < 0.001) and underwent prior PCI (56.1% vs. 0.0%, p < 0.001) (Table 2).
Survival
Survival was not significantly different between the two matched groups (HR = 0.90, 95% CI 0.61–1.32, p = 0.800) as shown in Fig. 2 (corresponding Kaplan–Meier analysis for the unmatched cohort (HR = 1.23, 95% CI 0.92–1.64, p = 0.167) is shown in Supp. Figure 2, and for no CAD vs. (I) 1- or 2-vessel disease, vs. (II) 3-vessel disease, vs. (III) prior MI, and vs. (IV) prior revascularization in Supp. Figure 3–6, respectively, accordingly without significant differences in survival). In addition, no significant difference in in-hospital mortality between LuTx patients with and without relevant CAD (8.2% vs. 8.2%, p > 0.999) could be detected (Table 3) (corresponding in-hospital analysis for the unmatched cohort is shown in Supp. Tab. 1–2).
Cardiovascular events following LuTx
Coronary angiography was required significantly more often in the matched CAD group (17.3% CAD vs. 5.1% non-CAD, p = 0.011). However, MI (7.1% CAD vs. 2.0% non-CAD, p = 0.170), PCI (5.1% vs. 1.0%, p = 0.212) and CABG (1% vs. 0%, p > 0.999) occurred numerically more frequently in the CAD group, but without reaching statistical significance. Stroke (2.0% vs. 6.1%, p = 0.279), atrial fibrillation (new onset) (15.3% vs. 23.5%, p = 0.206), dialysis (8.2% vs. 11.2%, p = 0.630) as well as cardiac arrest (6.1% vs. 11.2%, p = 0.310) were more frequent in the non-CAD group (Table 4, unmatched cohort is shown in Supp. Tab. 3–4) albeit without reaching statistical significance.
Cause of death following LuTx
Sepsis and infectious disease were the most common cause of death in LuTx patients (15.3% CAD vs. 16.3% non-CAD, p = 0.832), followed by chronic allograft dysfunction (11.2% vs. 6.1%, p = 0.118). Cardiovascular death occurred more often in the CAD group (7.1% vs. 2.0%, p = 0.078), albeit not reaching statistical significance. Further details on cause of death can be found in Table 5 (unmatched cohort is shown in Supp. Tab. 5–6).
Risk factors for in-hospital mortality in LuTx patients
The logistic regressions models revealed the following set of independent risk factors for in-hospital mortality: age at transplantation [years] (Odds Ratio [OR] 1.04, 95% confidence interval [CI] [1.01, 1.07], p = 0.009), elevated bilirubin [mg/dl] (OR 1.90, 95%CI [1.19, 3.03], p = 0.007), decreased forced vital capacity [% of reference] (OR 0.97, 95%CI [0.95, 0.99], p = 0.004), double lung transplantation (OR 2.04, 95%CI [1.04, 4.01], p = 0.039) and necessity of reoperation (OR 2.99, 95%CI [1.84, 4.87], p < 0.001) were associated with higher in-hospital mortality. Time since first transplantation [years] (OR 0.93, 95%CI [0.89, 0.97], p = 0.001) was associated with lower in-hospital mortality. However, relevant CAD (OR 1.30, 95%CI [0.55, 3.09], p = 0.547) did not emerge as a risk factor for in-hospital mortality. The results are summarized in Table 6.
Risk factors for all-cause mortality in LuTx patients
The following set of risk factors were independently related to all-cause mortality: Age at transplantation [years] (Hazard Ratio [HR] 1.02, 95%CI [1.01, 1.04], p < 0.001), elevated bilirubin [mg/dl] (HR 1.33, 95%CI [1.15, 1.54], p < 0.001), obstructive lung disease (HR 1.43, 95%CI [1.01, 2.02], p = 0.041), decreased forced vital capacity [% of reference] (HR 0.99, 95%CI [0.99, 1.00], p = 0.042) and necessity of reoperation (HR 3.51, 95%CI [2.97, 4.14], p < 0.001) were associated with higher all-cause mortality, while time since first transplantation [years] (HR 0.97, 95%CI [0.95, 0.99], p = 0.001) and double lung transplantation (HR 0.65, 95%CI [0.52, 0.80], p < 0.001) were associated with lower all-cause mortality. Similar to in-hospital mortality, relevant CAD (HR 0.96, 95%CI [0.71, 1.29], p = 0.788) was not associated with all-cause mortality. The results are summarized in Table 6.
Discussion
Even after control for multiple confounders, relevant CAD in lung transplant recipients was not associated with increased mortality in the current analysis. Considering various confounders by propensity score matching and Cox regression analysis, no influence on in-hospital and all-cause mortality was found. Cardiovascular events after transplantation were common, endorsing a rigorous management of cardiovascular risk factors and continuous cardiovascular monitoring as part of the structured follow-up of lung transplant recipients.
The 2021 ISHLT consensus document for the selection of lung transplant candidates lists mild to moderate CAD and revascularized CAD as a risk factor for an unfavourable outcome, but states that CAD should not be considered an absolute contraindication [25]. The current data do not provide evidence for an increased risk of mortality in a highly selected cohort with reduced long-term survival and with other predictors of outcome. Thus, patients with relevant CAD should not be per se excluded from lung transplantation but should rather thorough assessment of comorbidities. It is likely that patients with CAD have been otherwise deemed as good transplant candidates, which must be considered when interpreting our results.
Among 1,003 patients having undergone LuTx, 10.4% had relevant CAD in the present study. This is in line with previous investigations proving a relevant incidence of CAD in LuTx candidates [16,17,18,19,20,21]. This should not seem surprising since chronic lung disease and CAD share common risk factors, such as advanced age and smoking history. Furthermore, chronic respiratory disease itself is a risk factor for CAD most likely due to chronic inflammation and systemic hypoxia [31, 32]. Thus, the higher cardiovascular risk burden of LuTx candidates and the high incidence of CAD underlines the necessity of preoperative cardiovascular evaluation. In this context, coronary angiography is recommended since non-invasive tests are frequently limited by the advanced stages of lung disease [33, 34].
Overall, our findings contribute to an increasing body of literature studying the impact of CAD on outcomes after LuTx: Castleberry et al. analyzed 791 LuTx recipients between 1997 and 2010 and found no impact of revascularized CAD on risk for death. Preoperative PCI seemed to be superior to concurrent CABG in the context of LuTx [35]. Similarly, Kanaparthi et al. retrospectively investigated 468 patients undergoing single and double lung transplantation and found that preoperative (PCI n = 34, CABG n = 25) or intraoperative revascularization (CABG n = 29) did not negatively impact survival in LuTx patients [36]. In our analysis, preoperative and concurrent CABG were hardly performed, indicating a predominant revascularization strategy by PCI increasing statistical power and facilitating interpretation of results.
In line with our results previous, smaller cohort studies have not demonstrated survival differences between patients with no/mild or relevant CAD, keeping in mind different classifications and observation times [17, 19, 21, 24, 37]. One strength of the current analysis is the large number of analyzed patients as well as respective event rates allowing us to control for several confounders. We performed a detailed assessment in this large propensity-matched cohort, allowing an analysis of the impact of comorbidities and cardiovascular risk factors on outcomes.
Despite no difference in all-cause mortality, it remains unclear whether the presence of CAD is associated with cardiovascular events and cardiovascular deaths following LuTx. Chaikriangkrai et al. reported that relevant CAD was an independent predictor of cardiovascular events in their unmatched analysis (HR 20.32, 95% CI [5.79, 71.26], p < 0.001) concluding that CAD patients are at higher risk of non-fatal cardiovascular events [19]. Furthermore, Zanotti et al. showed that patients with mild-to-moderate CAD needed coronary revascularization more frequently than those without CAD [17]. Similarly, cardiovascular events after transplantation were more common and there was a trend towards more cardiovascular deaths in our cohort. However, significant differences between patients with and without CAD were present, making causality difficult to assess. After matching, differences in cardiovascular events differed without reaching statistical significance. Therefore, CAD may be considered a surrogate for cardiovascular outcome but may not be a risk factor for mortality in the context of a limited overall long-term survival of a lung transplant recipient. In this respect, vigorous and continuous cardiovascular follow-up in LuTx patients with CAD seems indicated. Furthermore, management of cardiovascular risk factors is of particular importance, as immunosuppressive drugs are associated with aggravation of hyperlipidaemia, arterial hypertension and diabetes mellitus [38].
In LuTx the effect of CAD on outcome may be limited by the reduced long-term survival and may be influenced by other outcome defining factors. Age at transplantation, elevated bilirubin, obstructive lung disease, decreased forced vital capacity, necessity of reoperation and early transplantation time were independent risk factors for all-cause mortality, while relevant CAD was not. Although age at transplantation is an anticipated risk factor, the overall survival disadvantage, despite superior in-hospital outcome, of patients with COPD may be explained by additional disease-specific morbidities and is in line with the recent ISHLT report [39]. Preoperative bilirubin can be interpreted as a surrogate parameter for right heart failure in the context of severe pulmonary hypertension. Patients with pulmonary arterial hypertension are at relevant risk for worse outcomes, particularly in the early post-transplantation period, this may be due to the complexities of lung transplantation and postoperative dysfunction of the left ventricle [40]. Furthermore, patients bridged to transplantation via ECMO are at risk for liver injury, as this complication is frequently irreversible and associated with high mortality [41]. While double lung transplantation was a significant risk factor for in-hospital mortality, most likely due to increased operative complexity, it was associated with reduced all-cause mortality. This highlights the long-term benefit of double lung transplantation irrespective of the higher perioperative complexity. However, the survival advantage is driven by a strong selection bias for double lung transplantation of patients deemed in a better overall condition.
This study analysed the outcome of LuTx patients over a study period of more than 20 years. During this time, management of LuTx recipients has advanced. For instance, the introduction of azithromycin therapy for the prevention and treatment of chronic lung allograft dysfunction and the implementation of the Lung Allocation Score in 2011 were significant changes. Finally, center experience contributes to improved outcomes and has evolved over time [42]. Consequently, it does not seem surprising, that in our Cox regression analysis a negative association between time since first transplantation and all-cause mortality was found. Furthermore, time since first transplantation was also negatively associated with in-hospital mortality, indicating improvements in candidate selection, perioperative management and postoperative care. With further improvements and increasing long-term survival after lung transplantation, the association between CAD with cardiovascular events and all-cause mortality may have to be reassessed.
As a final remark, the influence of organ transplantation on CAD is not sufficiently understood. On the one hand, the presence of a continuous inflammation due to alloimmunity has the potential to accelerate atherosclerosis and thereby CAD. On the other hand, the continuous immunosuppressive therapy is thought to decrease the progress of CAD. A clinical study investigating the rate of CAD progression in patients following solid organ transplantation compared to the overall population is direly needed to shed light on this question.
These results should be interpreted within their given limitations. Due to the inherent limitations such as a lack of randomization and blinding to outcomes, our study may be limited by unmeasured confounders and selection bias. We cannot rule out that a longer follow-up period may have led to significant differences in survival. With improvement of long-term outcome after transplantation due to advances in management of immunosuppression and chronic rejection, cardiovascular events might play a more important role in the future. Furthermore, we cannot rule out that some younger patients might have suffered from undetected CAD. However, due to the comprehensive cardiovascular assessment associated with transplant evaluation, this is very unlikely. To avoid overfitting not all potential risk factors for mortality could be included in the regression analysis. However, in contrast to previous studies many confounders were taken into account. Furthermore, due to the very small number of patients receiving CABG, a direct comparison with PCI could not be performed. On the other side the consistent use of PCI as the primary revascularization strategy facilitates interpretation of our results. In addition, patients excluded from LuTx due to cardiovascular evaluation had not been tracked in our system and are therefore unavailable for analysis. In this respect, it is likely that patients with CAD who were selected for transplantation were otherwise regarded as particularly good candidates potentially further affecting outcome analysis.
Conclusions
CAD is a frequent comorbidity in patients with end-stage lung disease requiring lung transplantation. In the present study, relevant CAD was not associated with short- and long-term survival. Cardiovascular events were more common in patients with CAD, likely due to due to differences in risk factor burden. After adjusting for confounders, no association of CAD and cardiovascular morbidity and mortality was found, indicating the need for rigorous management of cardiovascular risk factors after transplantation. The association of CAD and outcome after lung transplantation may need to be reassessed with improvement of management of transplant outcome defining factors and long-term survival, respectively.
Data Availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions and legal constraints.
References
GBD Chronic Respiratory Disease Collaborators (2020) Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 8(6):585–596. https://doi.org/10.1016/s2213-2600(20)30105-3
Nishimura K, Tsukino M (2000) Clinical course and prognosis of patients with chronic obstructive pulmonary disease. Curr Opin Pulm Med 6(2):127–132. https://doi.org/10.1097/00063198-200003000-00008
Glass DS, Grossfeld D, Renna HA, Agarwala P, Spiegler P, DeLeon J et al (2022) Idiopathic pulmonary fibrosis: Current and future treatment. Clin Respir J 16(2):84–96. https://doi.org/10.1111/crj.13466
Holm AM, Immer F, Benden C (2020) Lung allocation for transplant: The European perspective. Clin Transplant 34(7):e13883. https://doi.org/10.1111/ctr.13883
Lewis A, Koukoura A, Tsianos GI, Gargavanis AA, Nielsen AA, Vassiliadis E (2021) Organ donation in the US and Europe: The supply vs demand imbalance. Transplant Rev (Orlando) 35(2):100585. https://doi.org/10.1016/j.trre.2020.100585
Jones RM, Enfield KB, Mehrad B, Keeley EC (2014) Prevalence of obstructive coronary artery disease in patients undergoing lung transplantation: case series and review of the literature. Catheter Cardiovasc Interv 84(1):1–6. https://doi.org/10.1002/ccd.25261
Reed RM, Eberlein M, Girgis RE, Hashmi S, Iacono A, Jones S et al (2012) Coronary artery disease is under-diagnosed and under-treated in advanced lung disease. Am J Med 125(12):1228.e13-e22. https://doi.org/10.1016/j.amjmed.2012.05.018
Nations JA, Nathan SD (2009) Comorbidities of advanced lung disease. Mt Sinai J Med 76(1):53–62. https://doi.org/10.1002/msj.20086
Smith MC, Wrobel JP (2014) Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis 9:871–888. https://doi.org/10.2147/copd.S49621
Manoushagian S, Meshkov A (2014) Evaluation of solid organ transplant candidates for coronary artery disease. Am J Transplant 14(10):2228–2234. https://doi.org/10.1111/ajt.12915
Huiart L, Ernst P, Suissa S (2005) Cardiovascular morbidity and mortality in COPD. Chest 128(4):2640–2646. https://doi.org/10.1378/chest.128.4.2640
Kizer JR, Zisman DA, Blumenthal NP, Kotloff RM, Kimmel SE, Strieter RM et al (2004) Association between pulmonary fibrosis and coronary artery disease. Arch Intern Med 164(5):551–556. https://doi.org/10.1001/archinte.164.5.551
Nathan SD, Basavaraj A, Reichner C, Shlobin OA, Ahmad S, Kiernan J et al (2010) Prevalence and impact of coronary artery disease in idiopathic pulmonary fibrosis. Respir Med 104(7):1035–1041. https://doi.org/10.1016/j.rmed.2010.02.008
André S, Conde B, Fragoso E, Boléo-Tomé JP, Areias V, Cardoso J (2019) COPD and cardiovascular disease. Pulmonology 25(3):168–176. https://doi.org/10.1016/j.pulmoe.2018.09.006
International guidelines for the selection of lung transplant candidates (1998) The american society for transplant physicians (ASTP)/American Thoracic Society(ATS)/European Respiratory Society(ERS)/International society for heart and lung transplantation(ISHLT). Am J Respir Crit Care Med 158(1):335–339. https://doi.org/10.1164/ajrccm.158.1.15812
Choong CK, Meyers BF, Guthrie TJ, Trulock EP, Patterson GA, Moazami N (2006) Does the presence of preoperative mild or moderate coronary artery disease affect the outcomes of lung transplantation? Ann Thorac Surg 82(3):1038–1042. https://doi.org/10.1016/j.athoracsur.2006.03.039
Zanotti G, Hartwig MG, Castleberry AW, Martin JT, Shaw LK, Williams JB et al (2014) Preoperative mild-to-moderate coronary artery disease does not affect long-term outcomes of lung transplantation. Transplantation 97(10):1079–1085. https://doi.org/10.1097/01.Tp.0000438619.96933.02
Franz M, Siemeni T, Aburahma K, Yablonski P, Poyanmehr R, Avsar M et al (2022) Lung transplant and severe coronary artery disease: results from a single-centre experience. Eur J Cardiothorac Surg 62(2):ezac348. https://doi.org/10.1093/ejcts/ezac348
Chaikriangkrai K, Jyothula S, Jhun HY, Estep J, Loebe M, Scheinin S et al (2016) Impact of pre-operative coronary artery disease on cardiovascular events following lung transplantation. J Heart Lung Transplant 35(1):115–121. https://doi.org/10.1016/j.healun.2015.08.009
Sherman W, Rabkin DG, Ross D, Saggar R, Lynch JP 3rd, Belperio J et al (2011) Lung transplantation and coronary artery disease. Ann Thorac Surg 92(1):303–308. https://doi.org/10.1016/j.athoracsur.2011.04.021
Khandhar SJ, Althouse AD, Mulukutla S, Kormos R, Toma C, Marroquin O et al (2017) Postoperative outcomes and management strategies for coronary artery disease in patients in need of a lung transplantation. Clin Transplant 31(9):e13026. https://doi.org/10.1111/ctr.13026
Koprivanac M, Budev MM, Yun JJ, Kelava M, Pettersson GB, McCurry KR et al (2016) How important is coronary artery disease when considering lung transplant candidates? J Heart Lung Transplant 35(12):1453–1461. https://doi.org/10.1016/j.healun.2016.03.011
McKellar SH, Bowen ME, Baird BC, Raman S, Cahill BC, Selzman CH (2016) Lung transplantation following coronary artery bypass surgery-improved outcomes following single-lung transplant. J Heart Lung Transplant 35(11):1289–1294. https://doi.org/10.1016/j.healun.2016.05.029
Halloran K, Hirji A, Li D, Jackson K, Kapasi A, Meyer S et al (2019) Coronary artery disease and coronary artery bypass grafting at the time of lung transplantation do not impact overall survival. Transplantation 103(10):2190–2195. https://doi.org/10.1097/tp.0000000000002609
Leard LE, Holm AM, Valapour M, Glanville AR, Attawar S, Aversa M et al (2021) Consensus document for the selection of lung transplant candidates: an update from the international society for heart and lung transplantation. J Heart Lung Transplant 40(11):1349–1379. https://doi.org/10.1016/j.healun.2021.07.005
Allen JG, Arnaoutakis GJ, Weiss ES, Merlo CA, Conte JV, Shah AS (2010) The impact of recipient body mass index on survival after lung transplantation. J Heart Lung Transplant 29(9):1026–1033. https://doi.org/10.1016/j.healun.2010.05.005
Ehrsam JP, Benden C, Seifert B, Opitz I, Schneiter D, Weder W et al (2017) Lung transplantation in the elderly: Influence of age, comorbidities, underlying disease, and extended criteria donor lungs. J Thorac Cardiovasc Surg 154(6):2135–2141. https://doi.org/10.1016/j.jtcvs.2017.07.032
Schaffer JM, Singh SK, Reitz BA, Zamanian RT, Mallidi HR (2015) Single- vs double-lung transplantation in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis since the implementation of lung allocation based on medical need. JAMA 313(9):936–948. https://doi.org/10.1001/jama.2015.1175
Loor G, Brown R, Kelly RF, Rudser KD, Shumway SJ, Cich I et al (2017) Gender differences in long-term survival post-transplant: A single-institution analysis in the lung allocation score era. Clin Transplant. 31(3):e12889. https://doi.org/10.1111/ctr.12889
Klomjit N, Mehrnia A, Sampaio M, Bunnapradist S (2015) Impact of diabetes mellitus on survival outcome of lung transplant recipients: an analysis of OPTN/UNOS data. Clin Transpl 31:43–55
Adamson PD, Anderson JA, Brook RD, Calverley PMA, Celli BR, Cowans NJ, Crim C, Dixon IJ, Martinez FJ, Newby DE, Vestbo J, Yates JC, Mills NL (2018) Cardiac troponin i and cardiovascular risk in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol 72(10):1126–1137. https://doi.org/10.1016/j.jacc.2018.06.051
Kilic A, Merlo CA, Conte JV, Shah AS (2012) Lung transplantation in patients 70 years old or older: have outcomes changed after implementation of the lung allocation score? J Thorac Cardiovasc Surg 144(5):1133–1138. https://doi.org/10.1016/j.jtcvs.2012.07.080
Schiopu SRI, Zacherl M, Todica A, Bartenstein P, Milger K, Leuschner G, Munker D, Bauer M, Massberg S, Behr J, Neurohr C, Huber BC, Kneidinger N (2020) Feasibility and accuracy of SPECT myocardial perfusion imaging in end-stage lung disease. J Nucl Cardiol 27(3):903–911. https://doi.org/10.1007/s12350-019-01851-4
Wild J, Arrigo M, Isenring BD, Buergi U, Kurowski T, Schuurmans MM, Huber LC, Benden C (2015) Coronary artery disease in lung transplant candidates: role of routine invasive assessment. Respiration 89(2):107–111. https://doi.org/10.1159/000368368
Castleberry AW, Martin JT, Osho AA, Hartwig MG, Hashmi ZA, Zanotti G, Shaw LK, Williams JB, Lin SS, Davis RD (2013) Coronary revascularization in lung transplant recipients with concomitant coronary artery disease. Am J Transplant 13(11):2978–2988. https://doi.org/10.1111/ajt.12435
Kanaparthi J, Kashem MA, Suryapalam M, Zhao H, Brann S, Leotta E, Minakata K, Keshavamurthy S, Shigemura N, Toyoda Y (2020) Prior and perioperative revascularization does not affect survival in lung transplant patients. Ann Thorac Surg 109(6):1677–1683. https://doi.org/10.1016/j.athoracsur.2020.01.016
Makey IA, Sui JW, Huynh C, Das NA, Thomas M, Johnson S (2018) Lung transplant patients with coronary artery disease rarely die of cardiac causes. Clin Transplant. 32(9):e13354. https://doi.org/10.1111/ctr.13354
Yang K, Zhang M, Zhang B, Zhang Y, Zhao Q (2022) Systematic review and meta-analysis of calcineurin inhibitors on long-term prognosis of renal transplant patients. Transpl Immunol 75:101741. https://doi.org/10.1016/j.trim.2022.101741
Perch M, Hayes D Jr, Cherikh WS, Zuckermann A, Harhay MO, Hsich E, Potena L, Sadavarte A, Lindblad K, Singh TP, Stehlik J, International Society for Heart and Lung Transplantation (2022) The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-ninth adult lung transplantation report-2022; focus on lung transplant recipients with chronic obstructive pulmonary disease. J Heart Lung Transplant 41(10):1335–1347. https://doi.org/10.1016/j.healun.2022.08.007
Salman J, Ius F, Sommer W, Siemeni T, Kuehn C, Avsar M, Boethig D, Molitoris U, Bara C, Gottlieb J, Welte T, Haverich A, Hoeper MM, Warnecke G, Tudorache I (2017) Mid-term results of bilateral lung transplant with postoperatively extended intraoperative extracorporeal membrane oxygenation for severe pulmonary hypertension. Eur J Cardiothorac Surg 52(1):163–170. https://doi.org/10.1093/ejcts/ezx047
Schwarz S, Lang C, Harlander M, Štupnik T, Slambrouck JV, Ceulemans LJ, Ius F, Gottlieb J, Kuhnert S, Hecker M, Aigner C, Kneidinger N, Verschuuren EA, Smits JM, Tschernko E, Schaden E, Faybik P, Markstaller K, Trauner M, Jaksch P, Hoetzenecker K (2022) Gamma-glutamyltransferase is a strong predictor of secondary sclerosing cholangitis after lung transplantation for COVID-19 ARDS. J Heart Lung Transplant 41(10):1501–1510. https://doi.org/10.1016/j.healun.2022.06.020
Yang Z, Subramanian MP, Yan Y, Meyers BF, Kozower BD, Patterson GA, Nava RG, Hachem RR, Witt CA, Pasque MK, Byers DE, Kulkarni HS, Kreisel D, Itoh A, Puri V (2022) The impact of center volume on outcomes in lung transplantation. Ann Thorac Surg 113(3):911–917. https://doi.org/10.1016/j.athoracsur.2021.03.092
Funding
Open Access funding enabled and organized by Projekt DEAL. There was no external funding for this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical standards
All ethical standards were met in writing and submitting this correspondence.
Conflicts of interest
The authors declare no conflict of interests related to the submitted work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lüsebrink, E., Gade, N., Seifert, P. et al. The role of coronary artery disease in lung transplantation: a propensity-matched analysis. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02445-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02445-y