Abstract
Aims
The present study aimed to develop a comprehensive clinical- and echocardiography-based risk score for predicting cardiovascular (CV) adverse outcomes in patients with ischemic heart failure (IHF) and reduced left ventricular ejection fraction (LVEF).
Methods
This retrospective cohort study included 1341 hospitalized patients with IHF and LVEF < 50% at our hospital from 2009 to 2017. Cox regression models and nomogram were utilized to develop a comprehensive prediction model (C&E risk score) for CV mortality and CV-related events (hospitalization or death).
Results
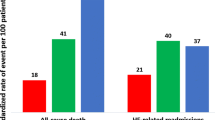
Over a median 26-month follow-up, CV mortality and CV events rates were 17.4% and 40.9%, respectively. The C&E risk score, incorporating both clinical and echocardiographic factors, demonstrated superior predictive performance for CV outcomes compared to models using only clinical or echocardiographic factors. Internal validation confirmed the stable predictive ability of the C&E risk score, with an AUC of 0.740 (95% CI 0.709–0.775, P < 0.001) for CV mortality and an AUC of 0.678 (95% CI 0.642–0.696, P < 0.001) for CV events. Patients were categorized into low-, intermediate-, and high-risk based on the C&E risk score, with progressively increasing CV mortality (5.3% vs. 14.6% vs. 31.9%, P < 0.001) and CV events (28.8% vs. 38.2% vs. 55.0%, P < 0.001). External validation also confirmed the risk score’s prognostic efficacy within additional IHF patient datasets.
Conclusion
This study establishes and validates the novel C&E risk score as a reliable tool for predicting CV outcomes in IHF patients with reduced LVEF. The risk score holds potential for enhancing risk stratification and guiding clinical decision-making for high-risk patients.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of heart failure (HF) rises with the aging population and improved survival rates among patients with heart diseases due to modern treatment innovation [1]. Ischemic heart disease plays a significant role in contributing to HF and holds crucial prognostic implications across the spectrum of this condition [2]. Prognostic assessment is of importance for risk stratification and optimizing patient care [3, 4]. To achieve this, ongoing efforts focus on developing prognostic risk scores with satisfactory clinical performance for HF patients [5]. In patients with ischemic HF (IHF), demographic, clinical, and hemodynamic factors collectively influence outcomes [6,7,8]. Previous studies have identified that left ventricular function, coronary stenosis distribution, and severity were pivotal survival determinants in patients with stable angina [9]. Additionally, distinct risk factors such as diabetes, prior myocardial infarction, hypertension, and male gender have surfaced as contributors to cardiovascular (CV) mortality or myocardial infarction in individuals with stable angina [7].
Transthoracic echocardiography (TTE) is crucial for evaluating cardiac function in daily clinical practice, providing comprehensive insights encompassing chamber dimensions, ventricular hypertrophy, regional wall movement anomalies, right-side performance, valvular function, systolic and diastolic function [10,11,12]. Several clinical studies emphasize the prognostic significance of echocardiographic parameters in HF patients with reduced LVEF, suggesting that utilizing a single echocardiographic prognostic marker or a combination of multiple markers could be a valuable approach for prognostic stratification [13]. In addition to conventional echocardiographic metrics, advanced technology-driven parameters, particularly speckle tracking derived global longitudinal strain (GLS), have shown supplementary prognostic potential, sometimes surpassing the predictive performance of LVEF in chronic systolic HF [14]. Despite these insights, the incremental role of jointly assessed clinical indexes and cardiac imaging-derived parameters, especially echocardiographic parameters, remains less explored in the prognostic framework for IHF patients with reduced LVEF [6]. To address this knowledge gap, the present study aimed to identify independent clinical and echocardiographic parameters, including both standard echocardiography metrics and GLS, for predicting major adverse cardiovascular outcomes in IHF patients with LVEF < 50%. Our objective involves developing and validating a comprehensive clinical- and echocardiography-based risk score (C&E risk score) tailored for the risk stratification of IHF patients with LVEF < 50%.
Methods
Study population
This retrospective cohort study comprised 1341 chronic HF patients with angiography-diagnosed ischemic heart disease and left ventricular systolic dysfunction (LVEF < 50%) admitted to our cardiology department from 2009 to 2017. Chronic HF diagnosis followed the current European Society of Cardiology guidelines [15]. Ischemic heart disease was confirmed clinically at baseline visit by coronary angiography defined stenosis of > 50% in ≥ 1 epicardial coronary artery with a visual reference lumen diameter of ≥ 2.5 mm, or patients had a history of myocardial infarction (MI), percutaneous coronary intervention (PCI), or coronary artery bypass graft (CABG) surgery [16]. De novo acute HF, malignancy, and other non-cardiac conditions limiting life expectancy to less than 1 year were excluded. HF patients with non-ischemic etiologies, including idiopathic dilated cardiomyopathy, valve heart disease, hypertensive heart disease, arrhythmias, conduction disturbances, chemotherapy-related cardiac dysfunction, myocarditis, infiltrative cardiomyopathy, hypertrophic cardiomyopathy, and other miscellaneous causes, were excluded. Figure 1 illustrates the study’s flowchart for developing a new prediction model.
Study flowchart. CV, cardiovascular; Echo, echocardiography; GLS, global longitudinal strain; IVSd, end‐diastolic interventricular septal thickness; LAVi, left atrial volume indexed to body surface area; LVEDD, left ventricular end‐diastolic dimension; LVEF, left ventricular ejection fraction; LVPWd, end‐diastolic posterior wall thickness; MAPSE, mitral annular plane systolic excursion; MR, mitral regurgitation; RAA, end‐systolic right atrial area; RVD, end‐diastolic mid-right ventricular diameter; sPAP, systolic pulmonary artery pressure; STI, speckle tracking imaging; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation
Ethics
The study was approved by the local Ethics Committee at the University of Würzburg and conducted in accordance to the Declaration of Helsinki. Written informed consent was obtained from patients and healthy volunteers.
Echocardiographic measures
A comprehensive TTE examination was performed according to the American Society of Echocardiography (ASE) recommendations (Vivid 7 or IE9, GE Vingmed Ultrasound, Horten, Norway) [17, 18]. Standard measurements were performed offline using the dedicated software (EchoPAC™, version 202, GE Vingmed Ultrasound AS, Horten, Norway). Thirteen standard echocardiography parameters together with GLS were initially evaluated. LV end-diastolic dimension (LVEDD), end-diastolic thickness of the posterior wall (LVPWd), and the septum (IVSd) were measured using M-mode in the parasternal LV long axis view. Right ventricular end-diastolic mid dimension (RVD) and end-systolic right atrial area (RAA) were measured in the RV-focused apical 4-chamber view. Left atrial volume (LAV) was measured in the LV-focused apical 4-chamber view at end systole. LAVi was calculated by dividing LAV by body surface area of subjects. LVEF was measured by using the Simpson biplane method from the apical 2- and 4-chamber view. Septal and lateral mitral annular plane systolic excursion (MAPSE) were respectively measured with a cursor respectively placed on the septal and lateral side of the mitral annulus from the LV-focused apical 4‐chamber view by M‐mode imaging. Average MAPSE value at septal and lateral annulus was calculated. Tricuspid annular plane systolic excursion (TAPSE) was measured in the RV-focused apical 4-chamber view by M-mode imaging. Pulsed-wave Doppler-derived mitral peak velocity of early (E) and atrial (A) diastolic filling was measured. Tissue-Doppler-derived early diastolic mitral annular velocity (e´) was acquired at the septal and lateral mitral annular sites and then septal, lateral, and average E/e´ ratio were calculated. Peak tricuspid regurgitation jet velocity (TRVmax) was measured with colour Doppler and continuous-wave Doppler. Systolic pulmonary artery pressure (sPAP) was derived from using the simplified Bernoulli equation in combination with an estimated right atrial pressure (RAP): sPAP = 4V2 + RAP, where V indicates the TRVmax. RAP was estimated through inferior vena cava diameter and its respiratory variation. Functional mitral regurgitation (MR) and tricuspid regurgitation (TR) were assessed, and their severity was graded as mild, moderate, or severe. Two-dimensional speckle tracking-derived longitudinal strain analysis was conducted offline in standard LV apical views (4-, 2-, and 3-chamber) with a frame rate ranging from 50 to 80 frames per second, spanning three consecutive cardiac cycles. This analysis was performed using the EchoPAC PC Software (version 202, GE Vingmed Ultrasound AS, Horten, Norway). Region of interest (ROI) was created by manually outlining the endocardial border on each LV apical view at the end-systolic frame. The system automatically tracked the tissue within the region and divided the myocardium into standard 18 LV segments. The trace analysis was automatically displayed after validating the tracking. GLS was automatically calculated by averaging the segmental strain of all 18 LV segments.
Outcomes
In this study, we initially conducted a thorough review of hospitalization records for each patient throughout the follow-up period. The primary reasons for hospitalization or death, along with the corresponding dates, were extracted. For patients without documented endpoints in hospital records, outcomes were assessed through telephone interviews with the patients or their family members. The outcome was further confirmed by contacting their general practitioners for detailed information. The primary endpoints were CV death and combined CV events included CV-related hospitalization or CV death. CV deaths were defined as deaths that result from an acute MI, sudden cardiac death, death due to HF, death due to CV procedures, death due to CV hemorrhage, death due to stroke, pulmonary embolism, peripheral arterial disease, or heart transplantation [19]. The CV causes of hospitalization included HF, acute/chronic coronary syndrome, uncontrolled hypertension, arrhythmia/atrial fibrillation, worsening renal function/acute renal dysfunction/cardiorenal syndrome, pulmonary embolism, or peripheral arterial disease.
Statistical analysis
Statistical analyses were performed using SPSS 28.0 (SPSS Inc., Chicago, IL, USA) and R Statistical Software (version 4.3.0; R Core Team 2022) along with Storm Statistical Platform (www.medsta.cn/software). Two-sided P values less than 0.05 were considered statistically significant in each statistical analysis.
Continuous variables were presented as mean (standard deviation) or median (interquartile range, IQR). Normal distribution of all continuous variables was checked by inspecting Q–Q plots and Shapiro–Wilk test. Continuous variables were compared by unpaired Student’s test or Mann–Whitney U-test. Differences cross three or four groups were compared using Kruskal–Wallis H test. Categorical or dichotomous variables were expressed as count and percent, and the differences among groups were compared using chi-square test.
The development of the prediction model was based on Cox proportional hazard regression analysis. In detail, a multiple imputation procedure utilizing random once imputation (a maximum of 50 iterations) was employed to address missing values in the major study variables identified (< 10%) prior to the primary analyses. Potential predictive variables associated with CV mortality were then explored through inter-group comparisons and univariate Cox regression analysis. Subsequently, variables showing statistical significance (P values < 0.05) were included in multivariable Cox regression analysis. Independent variables significantly associated with CV mortality were identified for the establishment of the final prediction model by multivariable Cox regression analysis, which was achieved using a stepwise backward elimination process based on likelihood ratio. Hazard ratio (HR) and 95% confidence interval (CI) were estimated. The variance inflation factor (VIF) was computed to assess the presence of potential multicollinearity among continuous variables. Multicollinearity was considered significant when the VIF > 5. In cases of multicollinearity, only one variable with the highest Wald value derived from univariate Cox regression analysis was retained for analysis. The nomogram representing the final multivariable Cox regression model results was constructed to visualize the developed prediction model, denoted as the C&E risk score, using the Regression Modelling Strategies R package (v6.6–0; Frank Harrell 2023) and the normogramFormula package (v1.2.0.0; Jing Zhang, Zhi Jin). To evaluate the discrimination ability of the models, receiver operating characteristic (ROC) curves based on Harrell’s concordance index (C-index) were employed. The area under the ROC curves (AUC) and 95% CI was assessed. To thoroughly assess the improvement in the prediction model's performance, we employed the net reclassification improvement (NRI) and integrated discrimination index (IDI). Each patient was assigned a risk score based on the established nomogram. Using tertiles of their respective risk scores, patients were categorized into low-risk, intermediate-risk, and high-risk groups. The prognostic significance of these three risk groups was evaluated through survival curves, and statistical differences were assessed using the log-rank test.
The R statistical software was utilized for both internal and external validation of the developed prediction model. Internal validation was performed by a bootstrap resampling approach with 1000 replications to assess the stability of the prediction model within the development cohort (i.e., the training set), comprising hospitalized IHF patients from 2009 to 2017. To evaluate the generalizability of the developed prediction model, external validation was conducted in three distinct patient sets: validation set 1 (IHF patients hospitalized in the year of 2018, excluding those rehospitalized in our hospital), validation set 2 (IHF patients treated with angiotensin receptor/neprilysin inhibitor (ARNIs) therapy hospitalized between 2016 and 2018), and validation set 3 (a combined cohort of IHF and non-IHF patients hospitalized in the year of 2018, excluding those rehospitalized in our hospital). The ROC curves, based on the C-index for predicting CV mortality and CV events risk, were then compared between the three validation sets and the training set.
Results
Baseline clinical characteristics of IHF patients with HFmrEF and HFrEF
Among 1341 IHF patients with LVEF < 50%, 595 (44.4%) had HFmrEF (LVEF 41–49%), and 746 patients (55.6%) had HFrEF (LVEF ≤ 40%, Table 1). The median clinical follow-up was 26 months (IQR 14–39). A total of 376 patients (28.0%) died, and 5 underwent heart transplantation (0.5%). CV mortality and CV events occurred at rates of 17.4% and 40.9%, respectively, with higher rates in HFrEF than HFmrEF groups (CV mortality: 20.8% vs. 13.3%, P < 0.001; CV events: 44.6% vs. 36.1%, P = 0.002). In this IHF cohort, baseline characteristics included a mean age of 70 ± 11 years, 79.6% males, 33.8% with NYHA class III or IV symptoms, 60.9% with a history of MI, 54.9% with PCI, and 28.4% had received CABG surgery. HFrEF patients, compared to HFmrEF, showed higher prevalence of NYHA class III or IV, higher systolic blood pressure, a lower prevalence of PCI, a higher prevalence of atrial fibrillation, hyperuricemia, renal dysfunction, peripheral vascular disease, chronic obstructive pulmonary disease (COPD), sleep disorders, and implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy with defibrillator (CRT-D) implantation. Laboratory findings displayed differences between HFmrEF and HFrEF, including markers of renal function, uric acid, total cholesterol, triglycerides, and N-terminal pro–B-type natriuretic peptide (NT-proBNP). Echocardiography data showed median LVEF of 39.0% (32.0–45.0)%, GLS of 9.9% (7.7–12.4)%, and significant differences in standard echocardiographic measures, including GLS, between the two groups.
Development of a comprehensive prediction model for CV mortality — C&E risk score
As shown in Table 2, initial assessment of clinical factors linked to CV mortality included age, NYHA class, diastolic blood pressure, PCI, atrial fibrillation, hyperuricemia, anemia, renal dysfunction, peripheral vascular disease, COPD, eGFR, creatinine, urea, C-reactive protein, uric acid, hemoglobin, total cholesterol, and NT-proBNP. These factors were identified based on a P value < 0.05 in inter-group comparisons between the no CV-death and CV-death groups, as well as in the univariate Cox regression model. Similarly, employing the same method, we identified, among the 14 observed echocardiographic parameters, that LAVi, RVD, RAA, LVEF, MAPSE, TAPSE, E/e´ ratio, sPAP, moderate to severe MR or TR, and GLS were significantly associated with CV mortality.
Multivariable Cox regression analysis with a stepwise backward elimination process, presented in Table 3, revealed that older age, COPD, lower eGFR and total cholesterol levels, higher uric acid levels, and Ln-transformed NT-proBNP were independent risk factors of CV mortality, and these variables were thus integrated into the clinical model. The echocardiographic independent risk factors eligible for the model included RVD, MAPSE, E/e´ ratio, sPAP, moderate to severe MR, and GLS. Subsequently, a comprehensive prediction model termed the C&E risk score was tested by integrating both the clinical and echocardiographic variables. Total cholesterol, E/e´ ratio, and GLS were excluded through the 4-step backward elimination process based on likelihood ratio. The final C&E risk score includes the following variables: age (HR 1.024, 95% CI 1.010–1.038, P = 0.001), COPD (HR 1.445, 95% CI 1.040–2.007, P = 0.028), eGFR (HR 0.989, 95% CI 0.983–0.995, P < 0.001), uric acid (HR 1.037, 95% CI 1.007–1.067, P = 0.014), NT-proBNP (HR 1.231, 95% CI 1.103–1.374, P < 0.001), RVD (HR 1.017, 95% CI 1.001–1.033, P = 0.041), MAPSE (HR 0.909, 95% CI 0.855–0.966, P = 0.002), sPAP (HR 1.010, 95% CI 1.000–1.019, P = 0.052), and moderate to severe MR (HR 1.432, 95% CI 1.052–1.949, P = 0.023).
Discrimination ability of the C&E risk score
The ROC curves based on the C-index underscored the discrimination ability of our prediction models (Fig. 2). The C&E risk score exhibited meaningful discriminatory power for CV mortality, with an AUC of 0.733 (95% CI 0.700–0.766, P < 0.001). The sensitivity was 0.709 (0.646–0.766), specificity was 0.675 (0.647–0.703), positive predictive value (PPV) was 0.316 (0.277–0.358), and negative predictive value (NPV) was 0.917 (0.895–0.934). Additionally, the C&E risk score demonstrated modest discriminatory performance for CV events, yielding an AUC of 0.639 (0.609–0.670, P < 0.001). The sensitivity for CV events was 0.691 (0.651–0.730), specificity was 0.531 (0.495–0.566), PPV was 0.505 (0.468–0.541), and NPV was 0.713 (0.675–0.749). The discriminatory power for CV mortality outperformed both the clinical model [AUC 0.707 (0.672–0.741); AUC difference 0.026, P = 0.003; NRI 13.4% (0.1–25.8%); IDI 0.019, P < 0.001] and the echo model [AUC 0.679 (0.642–0.717); AUC difference 0.054, P < 0.001; NRI 15.5% (0.2–27.6%); IDI 0.027, P < 0.001]. The discriminatory power for CV events likewise outperformed both the clinical model [AUC 0.625 (0.595–0.656); AUC difference 0.014, P = 0.035; NRI 9.7% (0.1–16.6%); IDI 0.021, P < 0.001] and the echo model [AUC 0.612 (0.581–0.643); AUC difference 0.028, P = 0.019; NRI 10.9% (3.2–18.7%); IDI 0.018, P < 0.001].
Comparison of discrimination abilities of the clinical model, the echocardiographic model, and the combined model (C&E risk score) using ROC curves based on Harrell’s concordance index (C-index). Notably, the combined prediction model (C&E risk score) demonstrates significantly enhanced discriminative capability for predicting CV mortality and CV events risk compared to the clinical or echocardiographic model in isolation. * P < 0.05 vs. C&E risk score model. AUC, area under the curve; CI, confidence interval; CV, cardiovascular; IHF, ischemic heart failure; ROC, receiver operating characteristic
To facilitate the prediction of 1-year, 2-year, and 3-year CV mortality in patients with IHF, we constructed a nomogram based on the C&E risk score. This nomogram offers a straightforward and intuitive means of estimating the probability of CV mortality by assigning values to each predictor, with each value corresponding to a score on the points scale (Fig. 3). The total score of each patient is calculated by summing individual scores of each predictor.
Nomogram based on the C&E risk score designed to predict 1-year, 2-year, and 3-year CV mortality in patients with IHF. This graphical tool offers an intuitive method for estimating the probability of CV mortality by assigning values to each predictor, with each value corresponding to a score on the points scale. The total score is calculated by summing these individual scores. COPD, chronic obstructive pulmonary disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; IHF, ischemic heart failure; MAPSE, mitral annular plane systolic excursion; MR, mitral regurgitation; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; RVD, end-diastolic mid-right ventricular diameter; sPAP, systolic pulmonary artery pressure
Risk classification of the IHF patients based on the C&E risk score for CV outcomes prediction
The median value of the C&E risk score in this cohort was 208 (180–234) points, and significantly higher in the HFrEF group compared to the HFmrEF group [217 (190–240) vs. 197 (171–223), P < 0.001]. Based on tertiles of the risk scores within this cohort, patients were categorized into three risk groups: low-risk (risk score: 0–188, n = 438), intermediate-risk (risk score: 189–224, n = 445), and high-risk (risk score: 225–350, n = 458). The outcomes of interest, including CV mortality (5.3% vs. 14.6% vs. 31.9%, P < 0.001), and the rate of CV events (28.8% vs. 38.2% vs. 55.0%, P < 0.001), exhibited a progressive increase across low-risk, intermediate-risk, and high-risk patients with IHF. Survival analysis using Kaplan–Meier curves further underscored these distinctions, revealing significant differences in cumulative CV-death free survival probabilities and CV-event hazard probabilities among these risk groups (both log rank P < 0.001; Fig. 4).
Cumulative CV-death free survival probabilities and CV-event hazard probabilities among low-risk, intermediate-risk, and high-risk patients with IHF through Kaplan–Meier curves. Patients were categorized into low-risk, intermediate-risk, and high-risk groups based on the tertiles of the risk scores within the cohort (low-risk: 0–188, n = 438; intermediate-risk: 189–224, n = 445; high-risk: 225–350, n = 458). The rates of CV mortality (5.3% vs. 14.6% vs. 31.9%) and CV events (28.8% vs. 38.2% vs. 55.0%) exhibit a progressive increase across the low-risk, intermediate-risk, and high-risk groups of patients with IHF and reduced left ventricular ejection fraction (all log rank P < 0.001). CV, cardiovascular
Comparisons of clinical and echocardiographic characteristics among low-risk, intermediated-risk, and high-risk patients with IHF revealed significant distinctions (Table S1). The high-risk patients were characterized by older age, a higher proportion of women, and an array of clinical factors. Furthermore, they presented with elevated serum levels of creatinine, urea, C-reaction protein, uric acid, and NT-proBNP. High-risk patients also showed a greater proportion of loop diuretics and digoxin use. Conversely, they had lower serum level of eGFR, hemoglobin, and total cholesterol, triglyceride, along with a reduced proportion of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) use.
Adjusted prognostic performance of the C&E risk score for predicting CV outcomes in IHF patients with HFmrEF and HFrEF
The independent prognostic performance of the C&E risk score for predicting CV outcomes was assessed, accounting for potential confounding factors such as age, sex, total cholesterol levels, use of ACEIs or ARBs, loop diuretics, digoxin, E/e´ ratio, and GLS in the analysis (Table 4). After adjusting for these potential confounding factors, the C&E risk score remained as an independent predictor of CV death and CV events in IHF patients. High-risk patients, as determined by the C&E risk score, experienced the most unfavorable CV outcomes. They faced a significantly increased risk of CV mortality (HR 4.567, 95% CI 2.615–7.976, P < 0.001), and an increased likelihood of experiencing CV events (HR 2.279, 95% CI 1.698–3.059, P < 0.001) compared to the low-risk group, as well as to the intermediate-risk group (CV mortality: HR 2.042, 95% CI 1.479–2.818, P < 0.001; CV events: HR 1.694, 95% CI 1.375–2.087, P < 0.001) among IHF patients, regardless of whether they fell into the HFmrEF or HFrEF subgroup.
Internal validation of the C&E risk score
Through internal validation employing 1000 bootstrap replicates, the determined median AUC of the C-index based ROC curves was 0.740 (95% CI 0.709 to 0.775, P < 0,001) for CV mortality prediction, and 0.678 (95% CI 0.642 to 0.696, P < 0.001) for CV events prediction. These results confirms the effective and stable discriminative capability of the C&E risk score in predicting CV mortality and CV events (Figure S1-A). The calibration curve further demonstrated that the nomogram model’s predictions was closely aligned with the actual observations, attesting to the reliability and accuracy of the C&E risk score in predicting CV mortality and CV events (Figure S1-B).
External validation of the C&E risk score
External validation also confirmed the prognostic efficacy of the established C&E risk score. Consistent predictive performance was observed for 1- and 3-year CV mortality, as well as 3-year CV events risk, not only in the original training dataset but also across diverse validation sets (Figures S2 and S3). This suggests the potential generalizability of the developed risk score for risk stratification among other HF patient populations (Table S2). Additionally, the risk classification of IHF based on the C&E risk score, categorized into low, intermediate, and high-risk groups, demonstrated predictive value for CV mortality and CV events within the validation sets (Figure S4).
Comparison of the C&E risk score with established Echo Heart Failure Score
We conducted a comparative analysis of the discrimination abilities between the C&E risk score and the published Echo Heart Failure Score (EHFS) [20] for predicting CV mortality in the development cohort (Figure S5). The EHFS incorporates five echocardiographic variables: end-systolic LV volume index ≥ 45 ml/m2, LAVi ≥ 84 ml/m2, mitral E-wave deceleration time ≤ 140 ms, TAPSE < 16 mm, and sPAP ≥ 45 mmHg, aiming to enhance risk prediction of death in systolic HF patients (LVEF < 45%). In the development cohort, the AUC of the C&E risk score for CV mortality prediction (AUC 0.733) was found to be significantly higher than that for EHFS (AUC 0.667; AUC difference 0.066 (95% CI 0.033–0.100), P < 0.001). This comparison underscores the superior discrimination ability of the C&E risk score in predicting CV mortality among IHF patients with LVEF < 50%.
Discussion
In this study, we established and validated a comprehensive prediction model for CV mortality and CV events risk in IHF patients with LVEF < 50% — C&E risk score. This score incorporates both clinical and echocardiographic predictors, including age, COPD, eGFR, uric acid, NT-proBNP, RVD, MAPSE, sPAP, and moderate to severe MR. The C&E risk score demonstrates significant discriminatory performance in predicting CV outcome in IHF patients, surpassing the predictive capabilities of either clinical or echocardiographic risk score alone. CV outcomes exhibited a proportional increase in C&E risk score-defined low-risk, intermediate-risk, and high-risk IHF patients with reduced LVEF. Internal and external validations further underscore its stability and the potential generalizability of the C&E risk score for risk stratification among other HF patient populations. This innovative C&E risk score might hold the potential to improve the risk assessment of IHF patients with reduced LVEF, help clinical decision-making of individualized monitoring and therapeutic strategies in these patients.
Several echocardiography-based prognostic scores have been developed for HF populations [20, 21]. Huttin et al. introduced the MEDIA echo score, featuring parameters such as sPAP > 40 mmHg, respiratory variation in inferior vena cava diameter > 0.5, E/e´ ratio > 9, and lateral mitral annular s´ < 7 cm/s. This score focuses on predicting all-cause mortality or cardiovascular readmission in HF patients with preserved LVEF (> 50%) [21]. The EHFS introduced by Carluccio et al. incorporating five echocardiographic variables, provides a simple stratification score strongly predictive of the risk of death in patients with chronic systolic HF (LVEF < 45%) [20]. Nevertheless, our findings demonstrate that for IHF patients with reduced systolic function (LVEF < 50%), the developed C&E risk score exhibits better prognostic performance than the EHFS. Stevens et al. reported the value of an echocardiographic score that incorporates five independent echocardiographic predictors, including left ventricular mass index, LAVi, MR, left ventricular outflow tract velocity–time integral, and diastolic dysfunction on predicting the subsequent development of HF in patients with stable coronary artery disease [22]. In general, there is still a knowledge gap in the assessment of CV-related adverse outcomes using an echocardiography-based scoring system in IHF patients with reduced LVEF. This clinical subgroup represents individuals at a notably high risk for HF. Furthermore, the incremental value of adding clinical parameters into the echocardiography-based risk score models for CV outcomes within this specific high-risk cohort of HF patients remains largely unexplored. In present study, we developed a comprehensive C&E risk score focused for IHF patients with reduced LVEF. Besides echocardiographic risk markers, clinical risk indicators, including age, COPD, eGFR, uric acid levels, and NT-proBNP were included in this score. These clinical indicators have previously been established as critical prognostic factors in ischemic heart disease [23, 24]. Our data unequivocally demonstrate that this combined model exhibits significantly enhanced discriminative capability for predicting adverse CV outcomes when compared to either clinical model or echocardiographic model. This novel C&E risk score might hold promise for improving risk assessment and management in these high-risk IHF patients.
The C&E risk score incorporates four key echocardiographic parameters (RVD, sPAP, MAPSE, and moderate to severe MR), each playing a distinct role in assessing cardiac function and providing valuable predictive information. RVD is indicative of RV dysfunction. Our dataset presents compelling evidence suggesting that the mid-cavity diameter of the RV stands out as the most robust predictor of CV mortality. sPAP offers a comprehensive assessment of pulmonary hypertension and cardiac function. Stern et al. reported an association between elevated sPAP (> 50 mmHg) and an increased risk of 1-year HF hospitalizations or all-cause mortality among patients undergoing cardiac resynchronization therapy [25]. The presence of moderate to severe functional MR is associated with significant LV remodeling and dysfunction, offering independent prognostic information in patients with ischemic LV dysfunction [26]. Research conducted by Rossi et al. suggests that severe functional MR can provide clinically relevant information irrespective of left ventricular function, extending its prognostic utility to both ischemic and non-ischemic dilated cardiomyopathy and varying degrees of HF severity [27]. MAPSE is indicative of global longitudinal function of the left ventricle [28, 29] and can be utilized to evaluate contractile reserve in patients with ischemic cardiomyopathy [30]. MAPSE has demonstrated its independence as a predictor of adverse outcomes across diverse patient populations [31,32,33]. It is noteworthy that GLS is a newer and more refined echocardiographic measure for assessing LV longitudinal function [34]. Clinical investigations have underscored the prognostic significance of GLS in a range of cardiovascular conditions [35], including chronic ischemic cardiomyopathy [36] and ST-segment elevation myocardial infarction [37]. Nonetheless, our analysis yielded an intriguing finding. Through the variable selection procedure, GLS did not emerge as a retained feature in our model. This may be attributed to GLS serving as an integrative parameter that potentially overlaps with other systolic function variables; further investigation is needed to clarify this issue.
Clinical implications
Our study holds key clinical implications. The developed C&E risk score, with its analytical edge, integrates diverse risk factors into a comprehensive model, surpassing the predictive capability of individual indicators. Although the AUC values fall below the threshold of 0.8, indicative of moderate predictive performance, the C&E risk score surpasses both clinical and echocardiographic models in predicting CV outcomes for this patient population. Internal and external validations have consistently demonstrated the stability and good generalizability of the risk stratification offered by the C&E score. This means that the score effectively identifies high-risk IHF patients, enabling proactive measures such as intensified monitoring and aggressive therapeutic interventions. Patients were categorized into low-, intermediate-, and high-risk groups based on tertiles of their risk scores. Our results confirm the utility of this grouping for risk stratification. Present findings demonstrate that this risk classification strategy aligns with clinical outcomes, effectively distinguishing between patients at varying risk levels. This approach might simplify communication for healthcare professionals and facilitate practical implications for tailoring interventions based on identified risk groups. Overall, our study supports the validity and utility of tertile-based risk classification as a valuable tool for personalized patient management.
Study limitations
Our study primarily focused on IHF patients with LVEF < 50%, limiting the generalizability of our findings to IHF patients with HFpEF. The data used in our retrospective analysis were derived from a single-center study, which may be related to selection bias and future external validity study is needed to verify our findings. Prospective studies are warranted to see if individualized therapy option could improve the CV outcome of high-risk IHF patients as defined by this score. Echocardiographic measurements vary among different operators and institutions, potentially affecting the accuracy and consistency of the C&E risk score. Standardized imaging protocols and quality control measures are thus essential to minimize the influence from these factors. Additionally, initial data collection did not include relevant information about coronary heart disease, such as angiography data and cardiac enzymology. Furthermore, we failed to obtain an independent cohort from another hospital or published studies with consistent MASPE measurements for validation. Future studies are warranted to further explore and clarify this issue. In light of this limitation, the validation was conducted in newly hospitalized IHF patients within our hospital in 2018.
It is crucial to acknowledge that the enrolled patients in our study experienced relatively high mortality rates and did not receive contemporary HF therapies in accordance with current guidelines. Approximately 8% of IHF patients in the development cohort received ARNIs, and none was prescribed sodium-glucose co-transporter 2 (SGLT2) inhibitors at baseline. The limited adoption of modern HF therapies raises valid concerns regarding the generalizability of our findings. Caution is warranted when interpreting mortality and associated outcomes due to the restricted use of advanced HF medications within our cohort. The retrospective nature of the data restricts our ability to assess the impact of initiating or switching to contemporary HF treatments on the C&E risk score. Notably, when compared to the development cohort (enrolled before 2018), CV mortality in IHF patients treated with ARNIs (validation set 2) was significantly lower (10.2% vs. 17.4%, P = 0.016), suggesting improved outcomes with the application of new HF medication. Nevertheless, the C&E risk score consistently demonstrated prognostic ability for CV mortality in this validation set, indicating potential applicability in the modern era of HF management. Prospective studies, including patients receiving modern HF therapies, are warranted to provide nuanced insights into the predictive performance of the C&E risk score in the evolving landscape of HF treatment strategies.
Conclusions
This study establishes and validates the novel C&E risk score as a reliable tool for predicting CV outcomes in IHF patients with reduced LVEF. The risk score holds potential for enhancing risk stratification and guiding clinical decision-making for high-risk patients.
Data Availability
The data that support the findings of this study are available on request from the corresponding author (PN). The data are not publicly available due to their containing information that could compromise the privacy of research participants.
References
Savarese G, Lund LH (2017) Global public health burden of heart failure. Card Fail Rev 3:7–11
Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WT, Braun OO, Savarese G, Dahlstrom U, Lund LH (2017) Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circ Heart Fail 10:e003875
Alba AC, Agoritsas T, Jankowski M, Courvoisier D, Walter SD, Guyatt GH, Ross HJ (2013) Risk prediction models for mortality in ambulatory patients with heart failure: a systematic review. Circ Heart Fail 6:881–889
Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN, Meta-Analysis Global Group in Chronic Heart F (2013) Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 34:1404–1413
Canepa M, Fonseca C, Chioncel O, Laroche C, Crespo-Leiro MG, Coats AJS, Mebazaa A, Piepoli MF, Tavazzi L, Maggioni AP, Investigators EHLTR (2018) Performance of prognostic risk scores in chronic heart failure patients enrolled in the European Society of Cardiology Heart Failure Long-Term Registry. JACC Heart Fail 6:452–462
Ginghina C, Bejan I, Ceck CD (2011) Modern risk stratification in coronary heart disease. J Med Life 4:377–386
Hjemdahl P, Eriksson SV, Held C, Forslund L, Nasman P, Rehnqvist N (2006) Favourable long term prognosis in stable angina pectoris: an extended follow up of the angina prognosis study in Stockholm (APSIS). Heart 92:177–182
Dunlay SM, Roger VL (2012) Gender differences in the pathophysiology, clinical presentation, and outcomes of ischemic heart failure. Curr Heart Fail Rep 9:267–276
Califf RM, Armstrong PW, Carver JR, D’Agostino RB, Strauss WE (1996) 27th Bethesda Conference: matching the intensity of risk factor management with the hazard for coronary disease events. Task Force 5. Stratification of patients into high, medium and low risk subgroups for purposes of risk factor management. J Am Coll Cardiol 27:1007–1019
Esmaeilzadeh M, Parsaee M, Maleki M (2013) The role of echocardiography in coronary artery disease and acute myocardial infarction. J Tehran Heart Cent 8:1–13
Marwick TH (2015) The role of echocardiography in heart failure. J Nucl Med 56(Suppl 4):31S-38S
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, Group ESCSD (2021) (2021) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42:3599–3726
Prastaro M, D’Amore C, Paolillo S, Losi M, Marciano C, Perrino C, Ruggiero D, Gargiulo P, Savarese G, Trimarco B, Perrone Filardi P (2015) Prognostic role of transthoracic echocardiography in patients affected by heart failure and reduced ejection fraction. Heart Fail Rev 20:305–316
Zhang KW, French B, May Khan A, Plappert T, Fang JC, Sweitzer NK, Borlaug BA, Chirinos JA, St John Sutton M, Cappola TP, Ky B (2014) Strain improves risk prediction beyond ejection fraction in chronic systolic heart failure. J Am Heart Assoc 3:e000550
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37:2129–2200
Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJ, Zamorano JL, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Valgimigli M, Claeys MJ, Donner-Banzhoff N, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Husted S, James SK, Kervinen K, Kristensen SD, Maggioni AP, Pries AR, Romeo F, Ryden L, Simoons ML, Steg PG, Timmis A, Yildirir A (2013) 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 34:2949–3003
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent LB, Cleveland O, Novara I, Rochester M, Bucharest R, St. Louis M (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17:1321–1360
Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, Morrow DA, Targum SL, Sila CA, Thanh Hai MT, Jaff MR, Joffe HV, Cutlip DE, Desai AS, Lewis EF, Gibson CM, Landray MJ, Lincoff AM, White CJ, Brooks SS, Rosenfield K, Domanski MJ, Lansky AJ, McMurray JJV, Tcheng JE, Steinhubl SR, Burton P, Mauri L, O’Connor CM, Pfeffer MA, Hung HMJ, Stockbridge NL, Chaitman BR, Temple RJ (2018) 2017 cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol 71:1021–1034
Carluccio E, Dini FL, Biagioli P, Lauciello R, Simioniuc A, Zuchi C, Alunni G, Reboldi G, Marzilli M, Ambrosio G (2013) The ‘Echo Heart Failure Score’: an echocardiographic risk prediction score of mortality in systolic heart failure. Eur J Heart Fail 15:868–876
Huttin O, Fraser AG, Lund LH, Donal E, Linde C, Kobayashi M, Erdei T, Machu JL, Duarte K, Rossignol P, Paulus W, Zannad F, Girerd N, Media KaRen I (2021) Risk stratification with echocardiographic biomarkers in heart failure with preserved ejection fraction: the media echo score. ESC Heart Fail 8:1827–1839
Stevens SM, Farzaneh-Far R, Na B, Whooley MA, Schiller NB (2009) Development of an echocardiographic risk-stratification index to predict heart failure in patients with stable coronary artery disease: the Heart and Soul study. JACC Cardiovasc Imaging 2:11–20
Rapsomaniki E, Shah A, Perel P, Denaxas S, George J, Nicholas O, Udumyan R, Feder GS, Hingorani AD, Timmis A, Smeeth L, Hemingway H (2014) Prognostic models for stable coronary artery disease based on electronic health record cohort of 102 023 patients. Eur Heart J 35:844–852
Spinar J, Spinarova L, Malek F, Ludka O, Krejci J, Ostadal P, Vondrakova D, Labr K, Spinarova M, Pavkova Goldbergova M, Benesova K, Jarkovsky J, Parenica J (2019) Prognostic value of NT-proBNP added to clinical parameters to predict two-year prognosis of chronic heart failure patients with mid-range and reduced ejection fraction - a report from FAR NHL prospective registry. PLoS ONE 14:e0214363
Stern J, Heist EK, Murray L, Alabiad C, Chung J, Picard MH, Semigran MJ, Ruskin JN, Singh JP (2007) Elevated estimated pulmonary artery systolic pressure is associated with an adverse clinical outcome in patients receiving cardiac resynchronization therapy. Pacing Clin Electrophysiol 30:603–607
Lancellotti P, Troisfontaines P, Toussaint AC, Pierard LA (2003) Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation 108:1713–1717
Rossi A, Dini FL, Faggiano P, Agricola E, Cicoira M, Frattini S, Simioniuc A, Gullace M, Ghio S, Enriquez-Sarano M, Temporelli PL (2011) Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 97:1675–1680
Hu K, Liu D, Herrmann S, Niemann M, Gaudron PD, Voelker W, Ertl G, Bijnens B, Weidemann F (2013) Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging 14:205–212
Grue JF, Storve S, Dalen H, Salvesen O, Mjolstad OC, Samstad SO, Torp H, Haugen BO (2018) Automatic measurements of mitral annular plane systolic excursion and velocities to detect left ventricular dysfunction. Ultrasound Med Biol 44:168–176
Magdy G, Hamdy E, Elzawawy T, Ragab M (2018) Value of mitral annular plane systolic excursion in the assessment of contractile reserve in patients with ischemic cardiomyopathy before cardiac revascularization. Indian Heart J 70:373–378
Rydberg E, Erhardt L, Brand B, Willenheimer R (2003) Left atrioventricular plane displacement determined by echocardiography: a clinically useful, independent predictor of mortality in patients with stable coronary artery disease. J Intern Med 254:479–485
Ozer PK, Govdeli EA, Demirtakan ZG, Nalbant A, Baykiz D, Orta H, Bayraktar BB, Baskan S, Umman B, Bugra Z (2022) The relation of echo-derived lateral MAPSE to left heart functions and biochemical markers in patients with preserved ejection fraction: Short-term prognostic implications. J Clin Ultrasound 50:593–600
Matos JD, Balachandran I, Heidinger BH, Mohebali D, Feldman SA, McCormick I, Litmanovich D, Manning WJ, Carroll BJ (2020) Mitral annular plane systolic excursion and tricuspid annular plane systolic excursion for risk stratification of acute pulmonary embolism. Echocardiography 37:1008–1013
Karlsen S, Dahlslett T, Grenne B, Sjoli B, Smiseth O, Edvardsen T, Brunvand H (2019) Global longitudinal strain is a more reproducible measure of left ventricular function than ejection fraction regardless of echocardiographic training. Cardiovasc Ultrasound 17:18
Iacoviello M, Puzzovivo A, Guida P, Forleo C, Monitillo F, Catanzaro R, Lattarulo MS, Antoncecchi V, Favale S (2013) Independent role of left ventricular global longitudinal strain in predicting prognosis of chronic heart failure patients. Echocardiogr - J Card 30:803–811
Bertini M, Ng ACT, Antoni ML, Nucifora G, Ewe SH, Auger D, Marsan NA, Schalij MJ, Bax JJ, Delgado V (2012) Global longitudinal strain predicts long-term survival in patients with chronic ischemic cardiomyopathy. Circ Cardiovasc Imaging 5:383–391
Holzknecht M, Reindl M, Tiller C, Reinstadler SJ, Lechner I, Pamminger M, Schwaiger JP, Klug G, Bauer A, Metzler B, Mayr A (2021) Global longitudinal strain improves risk assessment after ST-segment elevation myocardial infarction: a comparative prognostic evaluation of left ventricular functional parameters. Clin Res Cardiol 110:1599–1611
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF 01EO1504).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Dan Liu, Kai Hu, and Camilla Wagner. The first draft of the manuscript was written by Dan Liu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, D., Hu, K., Wagner, C. et al. Clinical value of a comprehensive clinical- and echocardiography-based risk score on predicting cardiovascular outcomes in ischemic heart failure patients with reduced ejection fraction. Clin Res Cardiol (2024). https://doi.org/10.1007/s00392-024-02399-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-024-02399-1