Abstract
Background
The diagnosis of heart failure with preserved ejection fraction (HFpEF) remains challenging. Recently, the HFpEF Stress Trial demonstrated feasibility and accuracy of non-invasive cardiovascular magnetic resonance (CMR) real-time (RT) exercise-stress atrial function imaging for early identification of HFpEF. However, no outcome data have yet been presented.
Methods
The HFpEF Stress Trial (DZHK-17) prospectively recruited 75 patients with dyspnea on exertion and echocardiographic preserved EF and signs of diastolic dysfunction (E/eʹ > 8). 68 patients entered the final study cohort and were characterized as HFpEF (n = 34) or non-cardiac dyspnea (n = 34) according to pulmonary capillary wedge pressure (HFpEF: PCWP rest: ≥ 15 mmHg stress: ≥ 25 mmHg). These patients were contacted by telephone and hospital charts were reviewed. The clinical endpoint was cardiovascular events (CVE).
Results
Follow-up was performed after 48 months; 1 patient was lost to follow-up. HFpEF patients were more frequently compared to non-cardiac dyspnea (15 vs. 8, p = 0.059). Hospitalised patients during follow-up had higher H2FPEF scores (5 vs. 3, p < 0.001), and impaired left atrial (LA) function at rest (p ≤ 0.002) and stress (p ≤ 0.006). Impairment of CMR-derived atrial function parameters at rest and during exercise-stress (p ≤ 0.003) was associated with increased likelihood for CVE. CMR-Feature Tracking LA Es/Ee (p = 0.016/0.017) and RT-CMR derived LA long axis strain (p = 0.003) were predictors of CVE independent of the presence of atrial fibrillation.
Conclusions
Left atrial function emerged as the strongest predictor for 4-year outcome in the HFpEF Stress Trial. A combination of rest and exercise-stress LA function quantification allows accurate diagnostic and prognostic stratification in HFpEF.
Clinicaltrials.gov: NCT03260621.
Graphical abstract

Similar content being viewed by others
Introduction
Heart failure with preserved or mildly reduced ejection fraction (HFpEF) accounts for more than half of the heart failure population [1]. The heterogenous pathophysiology and late symptom onset not only delay the diagnosis of HFpEF [2] but complicate targeted therapeutic decisions [3,4,5]. For early identification of diastolic dysfunction and decision making in uncertain cases, current guidelines recommend invasive right heart catheterization (RHC) including exercise-stress testing [6, 7]. Especially the identification of an early disease stage may critically advance efforts in the prevention or delay of cardiac remodelling and clinical deterioration from diastolic dysfunction [4, 5, 8,9,10]. The invasive nature of RHC as well as challenging examination conditions especially using echocardiography [11] during physiological exercise-stress have been impediments to the widespread adoption of exercise-stress protocols to the clinical routine.
Recently, the HFpEF Stress Trial [12] has demonstrated high diagnostic accuracy of cardiovascular magnetic resonance imaging (CMR) real-time (RT) exercise-stress testing at high temporal resolution for the non-invasive diagnosis of HFpEF [13]. While this diagnostic methodology has shown promise for the diagnosis of HFpEF, data on its prognostic implications are scarce. The medium-term follow-up of the HFpEF Stress trial [14] demonstrated the impact of atrial strain for prognostic assessment. Given slow disease progress in HFpEF and initial early diagnosis due to exercise-stress testing, the present long-term follow-up aimed for higher numbers in cardiovascular events (CVE) for improved statistical evaluation.
Methods
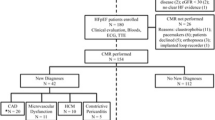
The study population of the HFpEF Stress Trial (NCT03260621) was followed up via telephone interviews and medical records 4 years after baseline recruitment [12]. The clinical endpoint was CVE including cardiovascular hospitalisation and mortality. 75 patients with exertional dyspnea (NYHA class ≥ II) and signs of diastolic dysfunction (E/e’ ≥ 8) and preserved left ventricular ejection fraction (LVEF) ≥ 50% on echocardiography were prospectively recruited between 08/2017 and 09/2019. Exclusion criteria comprised contraindications for CMR as well as other causes of dyspnea including pulmonary (forced expiratory volume in 1 s or vital capacity < 80% of the reference) or cardiac (coronary artery disease as defined by a luminal stenosis ≥ 50%, ≥ moderate valvular heart disease) conditions. Because of new diagnoses other than HFpEF on CMR imaging, 7 patients were excluded from final analysis, Fig. 1. All patients underwent RHC, echocardiography and CMR at rest and during exercise-stress using supine bicycle ergometry as previously reported [12]. Data acquisition was performed 3 min after surpassing a heart rate of 100 beats/min at 50–60 rpm using a 5 Watt increasing ramp protocol.
RHC assessments included right atrial and ventricular (RA/RV) as well as pulmonary artery (PA) and capillary wedge pressures (PCWP). The presence of HFpEF was defined according to PCWP of ≥ 15 mmHg at rest or ≥ 25 mmHg during exercise-stress on RHC assessments. Furthermore, HFpEF patients were classified as masked HFpEF if diagnosed during exercise-stress only or overt HFpEF if diagnosed at rest. Patients were classified as NCD if PCWP did not meet the criteria for HFpEF and the absence of other cardiovascular diseases on CMR, echocardiography and RHC. Echocardiography was performed for apical 2, 3, and 4 chamber views (CV) as well as short axis (SA) views. Doppler analysis was performed for assessments of aortic, mitral and tricuspid valve regurgitation (colour), aortic outflow and tricuspid regurgitation velocities (continuous wave) and E/e’ in septal and lateral position (pulsed wave and tissue). Speckle-tracking echocardiography (STE) was performed on 2 and 4 CV orientations. The study was approved by the local ethics committee at the University Medical Center Goettingen. All patients gave written informed consent before participation. The study was conducted according to the principles of the Helsinki Declaration and funded by the German Centre for Cardiovascular Research (DZHK-17) [15].
Cardiovascular magnetic resonance imaging
At the time of cardiac imaging, all patients were in stable sinus rhythm. Myocardial function was assessed with conventional ECG triggered cine imaging at rest and free breathing RT-CMR imaging at rest and during physiological exercise. Conventional imaging at rest was performed using balanced steady state free precession (bSSFP) sequences including 2-, 3- and 4- chamber views (CV) as well as a short axis (SA) stack covering the entire heart. Post processing included volumetric analyses and tissue characterisation using Medis (QMass®, Medical Imaging Systems, Leiden, Netherlands) as well as feature-tracking (FT) deformation imaging using TomTec (2D CPA MR, Cardiac Performance Analysis, TomTec Imaging Systems, Unterschleissheim, Germany) [16]. Global longitudinal strain (GLS) was assessed in long axis views, global circumferential and radial strains (GCS/GRS) were assessed in SA stacks, respectively. Atrial phasic function was analysed in 2 and 4 CV and classified according to reservoir (total strain Es), passive conduit (Ee) and active booster pump (Ea) function [17, 18], Fig. 2. RT-CMR imaging was performed at rest and during exercise-stress using a bSSFP sequence combined with an undersampled radial encoding scheme [13]. Left atrial (LA) and ventricular (LV) long axis strains (LAS) were assessed in the 2 and 4 CV using OsiriX MD (Pixmeo SARL, CH-1233 Bernex, Switzerland) [19, 20].
Feature-tracking and strain analysis. Top: On the left, left atrial (LA) end-systolic (ES) and -diastolic (ED) 2 and 4 chamber views (CV) with endocardial border tracking using cardiovascular magnetic resonance feature-tracking (CMR-FT). On the right, the corresponding strain curve of left atrial reservoir (εs), conduit (εe) and booster pump (εa) function for A a HFpEF patient without and B with cardiovascular event during follow-up. Bottom: Left atrial and ventricular (LV) long axis strain (LAS) assessment on a real-time CMR sequences shown at timespoints of ES and ED
Tissue characterisation was based on Modified Look-Locker Inversion recovery (MOLLI) sequences (pre- (5(3)3) and post-contrast (4(1)3(1)2) application) including septal and myocardial T1 times in one midventricular SA slice with subsequent calculation of extracellular volume (ECV) [21]. Late gadolinium enhancement (LGE) imaging was performed using inversion-recovery gradient echo sequences. Post-contrast images were obtained 10–20 min after the administration of gadolinium-based contrast agent (0.15 mmol/kg).
Statistical analyses
Categorical variables are reported as frequencies and corresponding percentages and were compared using the chi-squared test. Continuous variables were tested for normal distribution using the Shapiro–Wilk test, are reported as median with associated interquartile ranges (IQR) and were compared using the nonparametric Mann–Whitney U test. Patients characteristics are reported according to the occurrence of CVE. Predictors for the latter were identified from Cox regression analyses, the results of which are reported as hazard ratios (HR) with 95% confidence intervals (CI), as well as areas under the ROC curve (AUC) analyses reported with 95% CI. AUCs were compared using the method proposed by DeLong et al. [22]. A 2-tailed p value < 0.05 was considered statistically significant. Analyses were performed using SPSS version 26.0 (IBM, Armonk, New York, USA) and MedCalc version 18.2.1 (MedCalc Software bvba, Ostend, Belgium).
Results
Study population
The study population consisted of 68 patients (HFpEF n = 34, non-cardiac dyspnea (NCD) n = 34) from the HFpEF Stress Trial as shown in Fig. 1. One patient was lost to follow-up. Fifteen patients with HFpEF (heart failure n = 5, arrhythmia n = 8, hypertension n = 2) and eight with non-cardiac dyspnea (CAD including AMI, PCI and catheterisation n = 4, arrhythmia n = 3, heart failure n = 1) were hospitalised due to cardiovascular reasons (p = 0.059). Two patients had died, one of which due to cardiovascular reasons (heart failure hospitalisation followed by death). Cardiovascular hospitalisation and mortality are both considered in CVE. There were significantly more CVE in overt HFpEF (n = 8/15) compared to NCD (n = 8/26, p = 0.040). Baseline characteristics according to CVE are reported in Table 1. Hospitalised patients were slightly older (71 vs. 67, p = 0.030), while sex and cardiovascular risks factors were similar compared to patients without CVE during follow-up (p ≥ 0.345). Patients with CVE during follow-up had a higher H2FPEF [23] (heavy, 2 or more antihypertensive drugs, atrial fibrillation, pulmonary hypertension, elder age > 60, elevated filling pressures; 5 vs. 3, p < 0.001) but not HFA-PEFF (Heart Failure Association pretest assessment, echocardiography and natriuretic peptide, functional testing, final aetiology; 5 vs. 4, p = 0.103) scores.
On echocardiography patients with CVE showed no difference for E/e’ in echocardiography at rest (p = 0.132), the left atrial volume index (LAVI) was significantly increased (p = < 0.001) and AF was significantly more frequent in these patients (14/23, 61% with vs. 17/44, 16% without CVE, p < 0.001).
On RHC patients with CVE had higher PCWP and mean pulmonary artery (PA) pressures at rest and during exercise-stress (p ≤ 0.020).
Functional alterations
STE, FT- and RT-CMR deformation assessment parameters are reported in Table 2 according to CVE. LV function assessed by STE CMR-FT deformation imaging and RT-CMR LAS revealed no differences comparing patients with and without CVE during follow-up (p ≥ 0.146) with the exception of LV LAS at rest only, being impaired in patients with CVE (p = 0.028).
In contrast, LA function was impaired in patients with CVE. This included STE at rest (p < 0.001) and stress (p = 0.004), phasic function using CMR-FT (Es p < 0.001, Ee p < 0.001, Ea p = 0.002) as well as rest and exercise-stress RT-CMR derived LA EF (rest/stress: p = 0.002) and LA LAS (rest: p < 0.001, exercise-stress p = 0.006).
Prognostic implication
Hazard ratios for CVE and accuracies to predict CVE are reported in Table 3. An increase in the H2FPEF (HR 1.49, p = 0.006) but not in the HFA-PEFF (HR 1.29, p = 0.075) score was significantly associated with higher CVE rates. Visually, there was no LGE present. Tissue characterisation showed no association to outcome. STE, CMR-FT and RT-CMR LAS showed no significant association between LV function parameters and risk of CVE (p ≥ 0.171, AUC ≤ 0.64) except for LV LAS at rest (HR 0.84, p = 0.029).
Impaired atrial function at rest was associated with increased likelihood for CVE in Cox-regression analyses using STE, CMR-FT and RT-CMR (p ≤ 0.003) and during exercise-stress (p ≤ 0.004). This can further be appreciated from Kaplan–Meier plots after dichotomisation at the median, Fig. 3. These results remained significant in the subgroup of patients with invasively proven HFpEF (p ≤ 0.020). LA functional testing at rest using STE (p = 0.008), CMR-FT LA Es/Ee (p = 0.016/0.017) and RT-CMR derived LAS (p = 0.003) were independent predictors of CVE regardless of the presence of AF.
Cardiovascular events during follow-up. The graph shows the percentage of patients with cardiovascular events (CVE) in patients with A H2FPEF score ≤ 4 and ≥ 5 points, B cardiovascular magnetic resonance imaging (CMR) derived left atrial long axis strain (LA LAS) at rest above or below the median, C speckle tracking echocardiography (STE) derived left atrial reservoir strain (Es) at rest above or below the median and D CMR-feature tracking (FT) derived left atrial reservoir strain (Es) at rest above or below the median
The highest accuracy for the prediction of CVE was found for LA deformation imaging (AUC STE Es 0.86, CMR-FT Es 0.78, and RT CMR LAS 0.80), Fig. 4.
Accuracy to predict cardiovascular events. The figure displays the accuracy to predict cardiovascular events as areas under the ROC curve (AUC) for the H2FPEF score as well as cardiovascular magnetic resonance imaging (CMR) derived left atrial (LA) long axis strain (LAS), speckle tracking echocardiography (STE) derived reservoir strain (Es) and CMR-derived Es at rest
Subgroup analyses for NCD, masked and overt HFpEF revealed that only STE Es (p = 0.016/0.032) and LA LAS at rest (p = 0.037/0.017) have prognostic implications both in masked and overt HFpEF, Table 4. Prognostic implications remained significant in overt but not masked HFpEF independent of atrial fibrillation; LA LAS (overt HFpEF: HR 0.80, 95% CI 0.67–0.96, p = 0.018, masked HFpEF: HR 0.94, 95% CI 0.83–1.06, p = 0.287) and STE Es (overt HFpEF: HR 0.87, 95% CI 0.76–1.00, p = 0.042, masked HFpEF: HR 0.96, 95% CI 0.87–1.05, p = 0.334).
Discussion
The HFpEF Stress Trial has demonstrated high accuracy of RT-CMR physiological exercise-stress testing for the diagnosis of HFpEF [12]. The current analyses include the clinical 4-year follow-up of this cohort and demonstrate an association of LA function with outcome. Atrial functional failure determined by LA LAS during exercise-stress unmasks post capillary pulmonary hypertension for optimized non-invasive identification of HFpEF patients. In addition, atrial functional failure both at rest and during exercise-stress is associated with worse prognosis. Notwithstanding, LA dysfunction at rest provides the best accuracy for the prediction of CVE. Both STE Es and RT-CMR LA LAS yield high prognostic accuracy and were the only parameters with predictive value in both subgroups of overt and masked HFpEF; however, high quality STE for post-processing was only obtained in 80% of patients at rest and 68% during exercise-stress. Based on the previously published HFpEF Stress trial [12] and the current clinical follow-up data, we recommend that comprehensive CMR HFpEF assessments should incorporate atrial function quantification at rest and exercise-stress for the diagnostic and prognostic evaluation of HFpEF.
CMR for comprehensive assessment of cardiac pathology in HFpEF
CMR offers a comprehensive and non-invasive approach for the assessment of cardiac pathophysiology in HFpEF using tissue characterisation and function quantification [24,25,26]. The STIFFMAP Trial [27] demonstrated that CMR derived tissue characterisation independently predicts invasively assessed LV stiffness by pressure–volume loops. CMR deformation imaging allows the assessment of myocardial contractility and relaxation. CMR-FT derived LV GLS has been shown to correlate with invasively assessed LV relaxation Tau [28] and is associated with heart failure hospitalisation and mortality [29]. LA function has also been shown to be a sensitive imaging-biomarker for cardiac remodelling in HFpEF [30, 31] independent of atrial size [32] and associated with cardiovascular outcome [33]. CMR-FT atrial assessments offer differentiation of the three phases of atrial physiology, which can be attributed to atrial elasticity (reservoir and to some extent conduit function) and function parameters that contribute to LV filling (booster pump and to some extent conduit function) [17]. These phases have been shown to carry distinct physiological information with direct clinical and prognostic information. Atrial reservoir function, representing the collection of venous return during ventricular systole and elasticity of the atrium, is associated with cardiovascular mortality; for example, following acute myocardial infarction independent of ventricular function and tissue composition [34]. Passive atrial restoring forces and early diastolic ventricular filling are quantified by atrial passive conduit strain which has been shown to be associated with exercise intolerance in HFpEF [35] independent of LV stiffness and relaxation. LA active contractility (booster pump strain) has been reported to compensate for LV heart failure [36]. The finding that LA reservoir strain by both STE and CMR-FT provides high prognostic information regarding CVE may indicate that atrial elasticity by reducing pulmonary venous hypertension has stronger prognostic impact than LA function parameters contributing to LV filling (booster pump strain and to some extent conduit strain). We speculate of a prominent role of active atrial contractility during exercise as well as elasticity at rest in HFpEF pathophysiology. While exercise induced impaired active atrial contractility can precisely identify patients with HFpEF, impaired elasticity at rest identifies those patients at risk for hospitalisation due to congestion at rest or minimal levels of exercise at later disease stages. This is paralleled by data from Melenovsky et al. [37] who demonstrated that echocardiography derived active atrial contractility as defined by A’ from tissue velocity mapping during exercise was impaired and so a potential mechanism of cardio pulmonary congestion in HFpEF. Indeed, exercise-stress testing emerged as a cornerstone in the early diagnosis of HFpEF [6, 7]. Advances in CMR imaging introduced in the HFpEF Stress Trial [12, 13] demonstrated that RT-CMR imaging allows the transition of exercise-stress testing into the already broad spectrum of CMR imaging. Although RT-CMR does not allow for detailed deformation imaging assessment of the three atrial phases, our results show that it accurately reflects LA longitudinal function (and potentially increased active contractility during exercise), which allows for the identification of early stage HFpEF based on surrogate estimation (LV/LA LAS) of global LV and LA longitudinal strains [20]. This allows the translation of longitudinal deformation imaging to physiological exercise-stress assessments [12].
Rest and exercise-stress assessment for diagnostic and prognostic stratification in HFpEF
Even in the presence of exertional dyspnea, euvolemic HFpEF patients may present with normal natriuretic peptides and cardiac filling pressures at rest [6]. Exercise-stress induces atrial failure and congestion unmasking pathology in early stages of cardiac remodelling [38], thus allowing for the diagnosis of HFpEF. The HFpEF Stress Trial demonstrated assessment of exercise-induced atrial failure using LA LAS as the most precise parameter for the non-invasive identification of invasively proven HFpEF [12]. In the present study, atrial phasic function at rest, specifically reservoir function, and LA LAS at rest were identified as powerful predictors of CVE. Indeed, patients with advanced stages of cardiac remodelling and impaired atrial function at rest were more likely to be hospitalised during medium-term follow-up of 4 years. Based upon the presented data, for prognostic implications only, e.g., in known HFpEF, a shortened protocol for atrial function quantification including either conventional breath-hold bSSFP, novel free-breathing real-time CMR or echocardiography-based deformation imaging could be employed.
Importantly, in the present population, atrial function assessment (STE Es, CMR-FT Es/Ee and RT-CMR LA LAS) was associated with CVE independently of known AF in the medical history. Indeed, increased preload in diastolic dysfunction leads to atrial remodelling, dilatation and fibrillation [39]. AF burden further promotes atrial remodelling inducing progressive deterioration of atrial mechanics with subsequent worsening of cardiac haemodynamics [40, 41]. The distinct role of AF is also reflected in the H2FPEF score, with AF being the only factor accounting for 3 points [23]. Notwithstanding, atrial dysfunction is not limited to AF, the term atrial cardiomyopathy [42] has recently been introduced to describe intrinsic atrial dysfunction in cardiovascular diseases.
In our cohort at an early disease stage, 56% of HFpEF patients were identified by PCWP exercise-stress thresholds only [12]. Left atrial reservoir and conduit functions had higher diagnostic accuracy for CVE compared to booster (contractility) function. Indeed, reports in HFpEF indicate that changes in conduit function precede reduced atrial contractility, which initially shows an increase in active contractility in the early stages of diastolic dysfunction, compensating for increased LV filling pressures [43]. At later stages the contractility decreases as the disease progresses [44]. Notwithstanding, the assessment of a surrogate for global atrial function (LA LAS) on one hand side reliably detects an exercise induced atrial failure allowing the diagnosis of HFpEF and on the other hand side allows accurate risk stratification in the 4-year follow-up of our trial. The 2-year follow-up of the present population [14] had shown that a more in-depth assessment of the relative phasic function at rest provided the highest diagnostic accuracy. Indeed, as HFpEF tends to have slower progression of symptoms and disease severity compared to HFrEF, impaired phasic function may reflect atrial functional failure more accurately and thus may have the better short-term prognostic accuracy as these patients may suffer from CVE in the nearer future. For a long-term prognosis, overall atrial longitudinal function provides high diagnostic and prognostic accuracy. Importantly, to date, new therapeutic approach for HFpEF has demonstrated significant morbidity reduction [4], while it is important to note that delayed diagnosis may negate prognostic benefits [5, 45]. Atrial functional failure represents a composite of innate atrial functional loss [42] and the burden of LV congestion with increased filling pressures [46], the latter being closely related to symptom onset in HFpEF [47] highlighting its role in HFpEF.
In the current trial, an even longer period of follow-up (or higher patient numbers) may have led to a larger number of CVEs, and possibly a higher value for LV analyses for long-term prognosis prediction as previously demonstrated by Park et al. [29]. LV LAS at rest was the only ventricular parameter associated with CVE. This may again be due to the fact that patients included were at an early disease stage. This also highlights that progress in cardiac remodelling, once apparent in the LV, would significantly increase the risk of CVE. Future research, including a larger cohort of HFpEF patients further down the line of cardiac remodelling with a longer follow-up period, is required to fully understand the clinical significance and the prognostic impact of CMR-derived left atrial and ventricular function at rest and during exercise-stress. For the time being, the atrium reflects the ideal chamber for early diagnostic and prognostic assessments.
Study limitations
The HFpEF Stress Trial investigated a newly developed diagnostic test in an experienced CMR core-laboratory. Conclusions derived from this follow-up study, therefore, represents single center experience with a relatively small study population. Fifty-six percent of HFpEF patients were identified by exercise-stress testing only. As this may represent relatively early disease stages, a follow-up of 4 years may still be to short to allow for the development of adverse remodelling and the occurrence of CVE. Notwithstanding we were able to identify LA functional impairment at rest to be associated with the highest accuracy for CVE prediction in this population. Patient classification was based on PCWP and PA pressures only [7], while recently described invasively assessed criteria were not considered at the time of recruitment [48].
Conclusion
This follow-up study of the HFpEF Stress Trial demonstrates that left atrial function both at rest and during exercise-stress is associated with worse prognosis in HFpEF. However, atrial failure at rest is associated with disease progression and yields the highest prognostic value for the prediction of CVE. LA LAS allows easy and software independent approximation of LA longitudinal strain. Consequently, RT-CMR rest and exercise-stress LAS quantification enables both early diagnosis as well as prognostic estimation in HFpEF patients. A multi-center approach is warranted for further validation.
Data availability
The data underlying the findings is available at the imaging database of the German Centre for Cardiovascular Research (DZHK) and access will be granted to researchers that meet the criteria for access upon formal request.
Abbreviations
- AF:
-
Atrial fibrillation
- CMR:
-
Cardiovascular magnetic resonance
- EF:
-
Ejection fraction
- FT:
-
Feature tracking
- GLS:
-
Global longitudinal strain
- HFpEF:
-
Heart failure with preserved ejection fraction
- LA:
-
Left atrium
- LAS:
-
Long axis strain
- LV:
-
Left ventricle
- PCWP:
-
Pulmonary capillary wedge pressure
- RHC:
-
Right heart catheterization
- RT:
-
Real time
- STE:
-
Speckle-tracking echocardiography
References
McDonagh TA, Metra M, Adamo M et al (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 42(36):3599–3726
Paulus WJ, Tschöpe C, Sanderson JE et al (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550
Pitt B, Pfeffer MA, Assmann SF et al (2014) Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 370(15):1383–1392
Packer M, Butler J, Zannad F, et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients with Heart Failure and a Preserved Ejection Fraction: The EMPEROR-Preserved Trial. Circulation 2021.
Borlaug BA, Blair J, Bergmann MW et al (2022) Latent pulmonary vascular disease may alter the response to therapeutic atrial shunt device in heart failure. Circulation 144(16):1284–1294. https://doi.org/10.1161/CIRCULATIONAHA.121.056824
Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM (2010) Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 3(5):588–595
Pieske B, Tschöpe C, de Boer RA et al (2019) How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 40(40):3297–3317
Ravassa S, Trippel T, Bach D et al (2018) Biomarker-based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the Aldo-DHF trial. Eur J Heart Fail 20(9):1290–1299
Cleland JGF, Ferreira JP, Mariottoni B et al (2020) The effect of spironolactone on cardiovascular function and markers of fibrosis in people at increased risk of developing heart failure: the heart “OMics” in AGEing (HOMAGE) randomized clinical trial. Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa758
Edelmann F, Wachter R, Schmidt AG et al (2013) Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 309(8):781–791
Obokata M, Kane GC, Reddy YNV, Olson TP, Melenovsky V, Borlaug BA (2017) Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation 135(9):825–838
Backhaus SJ, Lange T, George EF et al (2021) Exercise-stress real-time cardiac magnetic resonance imaging for non-invasive characterisation of heart failure with preserved ejection fraction: the HFpEF stress trial. Circulation 143:1484–1498
Uecker M, Zhang S, Voit D, Karaus A, Merboldt K-D, Frahm J (2010) Real-time MRI at a resolution of 20 ms. NMR Biomed 23(8):986–994
Backhaus SJ, Rösel SF, Schulz A et al (2022) RT-CMR imaging for noninvasive characterization of HFpEF: medium-term outcomes of the HFpEF Stress trial. JACC Cardiovasc Imaging 15(5):943–945
Hoffmann J, Hanß S, Kraus M et al (2023) The DZHK research platform: maximisation of scientific value by enabling access to health data and biological samples collected in cardiovascular clinical studies. Clin Res Cardiol 112(7):923–941
Backhaus SJ, Metschies G, Zieschang V et al (2021) Head-to-head comparison of cardiovascular MR feature tracking cine versus acquisition-based deformation strain imaging using myocardial tagging and strain encoding. Magn Reson Med 85(1):357–368
Kowallick JT, Kutty S, Edelmann F et al (2014) Quantification of left atrial strain and strain rate using Cardiovascular Magnetic Resonance myocardial feature tracking: a feasibility study. J Cardiovasc Magn Reson 16:60
Schmidt-Rimpler J, Backhaus SJ, Hartmann FP et al (2023) Impact of temporal and spatial resolution on atrial feature tracking cardiovascular magnetic resonance imaging. Int J Cardiol. 2023:131563. https://doi.org/10.1016/j.ijcard.2023.131563
Schuster A, Backhaus SJ, Stiermaier T et al (2019) Fast manual long-axis strain assessment provides optimized cardiovascular event prediction following myocardial infarction. Eur Heart J Cardiovasc Imaging 20(11):1262–1270
Backhaus SJ, Rösel SF, Stiermaier T et al (2022) Left-atrial long-axis shortening allows effective quantification of atrial function and optimized risk prediction following acute myocardial infarction. Eur Heart J Open 2(5):053
Backhaus SJ, Lange T, Beuthner BE et al (2020) Real-time cardiovascular magnetic resonance T1 and extracellular volume fraction mapping for tissue characterisation in aortic stenosis. J Cardiovasc Magn Reson 22(1):46
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837
Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA (2018) A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 138(9):861–870
Backhaus SJ, Schuster A (2018) Atrial strain assessment in left ventricular diastolic dysfunction. JACC Cardiovasc Imaging 11(1):154
Backhaus SJ, Schuster A (2022) Atrial functional assessment at rest and during exercise stress in left ventricular diastolic dysfunction. Eur Heart J 43(36):3493–3494
Schulz A, Schuster A (2022) Visualizing diastolic failure: non-invasive imaging-biomarkers in patients with heart failure with preserved ejection fraction. EBioMedicine 86:1069
Rommel K-P, von Roeder M, Latuscynski K et al (2016) Extracellular volume fraction for characterization of patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 67(15):1815–1825
Ito H, Ishida M, Makino W et al (2020) Cardiovascular magnetic resonance feature tracking for characterization of patients with heart failure with preserved ejection fraction: correlation of global longitudinal strain with invasive diastolic functional indices. J Cardiovasc Magn Reson 22(1):42
Park JJ, Park J-B, Park J-H, Cho G-Y (2018) Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol 71(18):1947–1957
Schulz A, Lange T, Evertz R et al (2023) Sex-specific impairment of cardiac functional reserve in HFpEF. JACC Adv 2(4):100327
Backhaus SJ, Lange T, Schulz A et al (2023) Cardiovascular magnetic resonance rest and exercise-stress left atrioventricular coupling index to detect diastolic dysfunction. Am J Physiol 324(5):H686–H695
Santos ABS, Kraigher-Krainer E, Gupta DK et al (2014) Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail 16(10):1096–1103
Thomas L, Abhayaratna WP (2017) Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging 10(1):65–77
Schuster A, Backhaus SJ, Stiermaier T et al (2019) Left atrial function with mri enables prediction of cardiovascular events after myocardial infarction: insights from the AIDA STEMI and TATORT NSTEMI Trials. Radiology 293(2):292–302. https://doi.org/10.1148/radiol.2019190559
von Roeder M, Rommel K-P, Kowallick JT et al (2017) Influence of left atrial function on exercise capacity and left ventricular function in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 10:e005467
Backhaus SJ, Stiermaier T, Lange T et al (2019) Atrial mechanics and their prognostic impact in Takotsubo syndrome: a cardiovascular magnetic resonance imaging study. Eur Heart J Cardiovasc Imaging 20(9):1059–1069
Melenovsky V, Borlaug BA, Rosen B et al (2007) Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 49(2):198–207
Zakeri R, Moulay G, Chai Q et al (2016) Left atrial remodeling and atrioventricular coupling in a canine model of early heart failure with preserved ejection fraction. Circ Heart Fail 9(10):e003238
Abhayaratna WP, Seward JB, Appleton CP et al (2006) Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 47(12):2357–2363
Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA (2020) Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol 76(9):1051–1064
Patel RB, Lam CSP, Svedlund S et al (2021) Disproportionate left atrial myopathy in heart failure with preserved ejection fraction among participants of the PROMIS-HFpEF study. Sci Rep 11(1):4885
Guichard J-B, Nattel S (2017) Atrial cardiomyopathy: a useful notion in cardiac disease management or a passing fad? J Am Coll Cardiol 70(6):756–765
Bianco CM, Farjo PD, Ghaffar YA, Sengupta PP (2020) Myocardial mechanics in patients with normal LVEF and diastolic dysfunction. JACC 13(1):258–271
Rosca M, Lancellotti P, Popescu BA, Piérard LA (2011) Left atrial function: pathophysiology, echocardiographic assessment, and clinical applications. Heart (British Cardiac Society) 97(23):1982–1989
Schuster A, Schulz A, Lange T et al (2023) Concomitant latent pulmonary vascular disease leads to impaired global cardiac performance in heart failure with preserved ejection fraction. Eur J Heart Fail 25(3):322–331
Maeder MT, Thompson BR, Brunner-La Rocca H-P, Kaye DM (2010) Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol 56(11):855–863
Hasenfuß G, Hayward C, Burkhoff D et al (2016) A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. The Lancet 387(10025):1298–1304
Baratto C, Caravita S, Soranna D et al (2021) Current limitations of invasive exercise hemodynamics for the diagnosis of heart failure with preserved ejection fraction. Circ Heart Fail 14(5):e007555
Funding
Open Access funding enabled and organized by Projekt DEAL. German Centre for Cardiovascular Research (DZHK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Backhaus, S.J., Schulz, A., Lange, T. et al. Real-time cardiovascular magnetic resonance imaging for non-invasive characterisation of heart failure with preserved ejection fraction: final outcomes of the HFpEF stress trial. Clin Res Cardiol 113, 496–508 (2024). https://doi.org/10.1007/s00392-023-02363-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02363-5