Abstract
Background
Right ventricular-to-pulmonary artery (RV–PA) coupling has recently been shown to be associated with outcome in valvular heart disease. However, longitudinal data on RV dysfunction and reverse cardiac remodeling in patients following transcatheter edge-to-edge mitral valve repair (M-TEER) are scarce.
Methods
Consecutive patients with primary as well as secondary mitral regurgitation (MR) were prospectively enrolled and had comprehensive echocardiographic and invasive hemodynamic assessment at baseline. Kaplan–Meier estimates and multivariable Cox-regression analyses were performed, using a composite endpoint of heart failure hospitalization and death.
Results
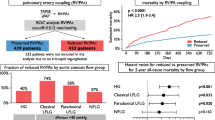
Between April 2018 and January 2021, 156 patients (median 78 y/o, 55% female, EuroSCORE II: 6.9%) underwent M-TEER. On presentation, 64% showed impaired RV–PA coupling defined as tricuspid annular plane systolic excursion to pulmonary artery systolic pressure (TAPSE/PASP) ratio < 0.36. Event-free survival rates at 2 years were significantly lower among patients with impaired coupling (57 vs. 82%, p < 0.001), both in patients with primary (64 vs. 91%, p = 0.009) and secondary MR (54 vs. 76%, p = 0.026). On multivariable Cox-regression analyses adjusted for baseline, imaging, hemodynamic, and procedural data, TAPSE/PASP ratio < 0.36 was independently associated with outcome (adj.HR 2.74, 95% CI 1.17–6.43, p = 0.021).
At 1-year follow-up, RV–PA coupling improved (TAPSE: ∆ + 3 mm, PASP: ∆ − 10 mmHg, p for both < 0.001), alongside with a reduction in tricuspid regurgitation (TR) severity (grade ≥ II: 77–54%, p < 0.001).
Conclusions
TAPSE/PASP ratio was associated with outcome in patients undergoing M-TEER for primary as well as secondary MR. RV–PA coupling, alongside with TR severity, improved after M-TEER and might thus provide prognostic information in addition to established markers of poor outcome.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Transcatheter edge-to-edge mitral valve repair (M-TEER) has recently been introduced as treatment option for patients with significant mitral regurgitation (MR) [1, 2]. Based on emerging evidence of favorable long-term durability, M-TEER is increasingly used in clinical practice [3, 4]. However, pre-procedural risk assessment remains challenging and longitudinal data on reverse cardiac remodeling and outcome are limited.
Previous studies have highlighted the prognostic significance of right ventricular-to-pulmonary artery (RV–PA) coupling, as a more robust measurement of RV dysfunction, in patients with heart failure [5], pulmonary hypertension [6], and/or valvular heart disease [7]. In a retrospective analysis of the EuroSMR registry, Karam et al. [8] have recently demonstrated that impaired RV–PA coupling, defined as tricuspid annular plane systolic excursion (TAPSE)-to-PA systolic pressure (PASP) ratio, was an important predictor of mortality in patients undergoing M-TEER for secondary MR. However, according to the newly issued ACC/AHA guidelines [2], M-TEER is not only considered for patients with secondary MR and poor left-ventricular (LV) systolic function, but also received a IIa recommendation for primary MR. Yet, optimal patient selection is crucial, especially in light of conflicting results from large randomized trials [4, 9].
Accordingly, for the first time, our prospective all-comer study of consecutive patients undergoing both comprehensive echocardiographic and invasive hemodynamic assessment prior to M-TEER aimed to investigate the impact of RV–PA coupling on outcome and reverse RV remodeling in patients with primary as well as secondary MR.
Methods
Study design
This prospective, observational study was conducted at the Medical University of Vienna, Austria, a university-affiliated tertiary care center with a multimodality imaging laboratory and a high-volume cardiac catheterization unit. Consecutive patients with significant MR scheduled for M-TEER were recruited. All cases were discussed individually by our local multidisciplinary Heart Team. The investigation conforms to the principles outlined in the Declaration of Helsinki, and the study protocol was approved by our Institutional Review Board (identifier: EK 1881/2012, amended version 01/2021). Written informed consent was obtained in all patients prior to study enrollment.
Echocardiography
Comprehensive echocardiographic assessments, including transesophageal echocardiography (TEE), were performed by board-certified cardiologists using high-end scanners (e.g., Vivid E95, GE Healthcare, Chicago, IL, USA). Cardiac chamber size was assessed according to current recommendations [10]. LVEF was calculated using the biplane Simpson’s method. RV systolic function was assessed by TAPSE, fractional area change (FAC), systolic movement of the RV lateral wall using tissue Doppler imaging (S’), and 2D speckle tracking echocardiography, based on the current guidelines [11]. RV dysfunction was defined by TAPSE < 17 mm, RV FAC < 35%, S’ < 9.5 cm/s, and RV free-lateral-wall strain > − 20% (less negative values indicate impaired function). These cut-off values were chosen according to previous studies [12, 13] and current recommendations [10, 14]. PASP was calculated by adding the peak tricuspid regurgitation (TR) systolic gradient to the estimated central venous pressure. RV–PA coupling was assessed using the TAPSE/PASP ratio. Valvular heart disease was quantified using an integrated approach as recommended in the respective guidelines [15, 16]. Severity of MR was determined using morphological criteria and jet direction (myxomatous degeneration, leaflet prolapse/flail), as well as quantification by vena contracta width, estimated regurgitant volume, and the effective regurgitant orifice area. In accordance with the previously published literature [3, 16], we applied a scale ranging from 1 to 4 in order to define MR severity: grade 1 indicates “mild”, 2 “moderate”, 3 “moderate-to-severe”, and 4 refers to “severe” MR. Sonographers were blinded to procedural and outcome data.
Invasive hemodynamics
Invasive hemodynamic assessment was performed routinely in study participants immediately prior to M-TEER. Hemodynamic measurements were performed using a 7F Swan-Ganz catheter (Edwards Lifesciences GmbH, Austria) via a femoral access. Pressures were documented as average of eight measurements over eight consecutive heart cycles using CathCorLX (Siemens AG, Berlin and Munich, Germany). In addition to PA wedge pressure (PAWP), the systolic (sPAP), diastolic (dPAP) and mean PA (mPAP) pressures were documented. TAPSE/sPAPinv ratio was calculated using the invasively measured sPAP. Cardiac output (CO) was measured by Fick’s method or thermodilution. If both were available, Fick’s method was preferred. Furthermore, the transpulmonary gradient (TPG) and diastolic pulmonary vascular pressure gradient (DPG) were calculated according to the current guidelines [17]. TPG was computed by subtracting PAWP from mPAP; DPG was calculated as the difference between dPAP and PAWP during pull-back; pulmonary vascular resistance (PVR) was calculated by dividing TPG by CO.
TEER procedures
All procedures were performed under general anesthesia with TEE and fluoroscopic guidance [18]. In brief, the edge-to-edge mitral repair system (MitraClip™ NTR, XTR, or PASCAL™) was introduced through the femoral vein and advanced to the MV by crossing the inter-atrial septum. Up to three edge-to-edge devices were placed into the MV to maximally reduce MR. Concomitant transcatheter edge-to-edge tricuspid valve repair (T-TEER) was performed in patients with significant TR by discretion of our Heart Team.
Outcome measures
Patients were prospectively followed after M-TEER in a dedicated outpatient clinic at 3 months, 12 months, and yearly thereafter. The primary outcome measure was a combined endpoint consisting of HF hospitalization (HFH) and death. HFH was defined as inpatient admission with clinical signs and symptoms of HF and requirement for intravenous diuretic treatment. Endpoints were ascertained by follow-up visits, state-wide electronic hospital charts, and direct patient phone calls. Mortality data were obtained via the National Registry of Deaths (Statistics Austria). The internal adjudication committee, consisting of CH and JM, blinded to echocardiography and procedural data, confirmed all endpoints.
Statistical analysis
Continuous data are presented as median (interquartile range [IQR]), categorical variables as total numbers and percentages, respectively. Comparisons between groups were performed using either Chi-squared or Fisher’s exact tests for categorical variables or Wilcoxon rank-sum tests for continuous variables, as appropriate. For correlation analyses, Spearman’s coefficients were used. In line with the previous studies [19, 20], the optimal cut-off for impaired RV–PA coupling was defined as TAPSE/PASP ratio < 0.36. Kaplan–Meier curves were plotted and the Log-rank test was used to estimate differences between survival curves. Cox-regression models were calculated to investigate the association between RV–PA coupling and the composite endpoint of HFH and death. In addition to crude analyses, we pre-defined three multivariable models: Model (A) adjusted for the EuroSCORE II, Model (B) adjusted for the EuroSCORE II and logarithmized NT-proBNP levels, and Model (C) adjusted for clinical and imaging variables with a significant impact on outcome using a stepwise multivariable model, which included all variables with a significant influence on an univariable level. To account for potential bias, we then repeated outcome analysis in patients who underwent isolated M-TEER in absence of concomitant T-TEER. Comparisons between baseline and follow-up measures were performed using either Chi-squared or Fisher’s exact tests or Wilcoxon signed-rank tests including and excluding patients who had concomitant T-TEER, respectively. A two-sided p value < 0.05 was considered statistically significant. All analyses were performed using SPSS 27 (IBM SPSS, USA).
Results
Baseline characteristics
In total, 163 consecutive patients scheduled for M-TEER were screened between April 2018 and January 2021. TAPSE/PASP ratio was available in 156 (96%) patients, who were included in the final analysis. Baseline characteristics and concomitant medication are summarized in Table 1. Impaired RV–PA coupling was present in 100 (64%) individuals. Compared to patients with normal coupling, TAPSE/PASP ratio was significantly associated with a higher EuroSCORE II (8.9 vs. 5.0%, p = 0.001), elevated NT-proBNP levels (4149 vs. 2612 pg/mL, p = 0.008), and impaired renal function (Creatinine: 1.3 vs. 1.2 mg/dL, estimated glomerular filtration rate [eGFR]: 47 vs. 52 mL/min/1.73m2, p for both < 0.041) at baseline. Impaired coupling was further associated with coronary artery disease (59 vs. 41%, p = 0.031), previous coronary artery bypass grafting (24 vs. 11%, p = 0.043), as well as pacemaker implantation (38 vs. 21%, p = 0.033).
Imaging data and invasive hemodynamics
Table 2 summarizes echocardiographic and Table 3 summarizes hemodynamic data at baseline. Patients with impaired RV–PA coupling showed significantly lower RV FAC (34.9 vs. 40.5%), S’ (9 vs. 10 cm/s), and higher RV global longitudinal strain (− 17 vs. − 20%), indicating worse RV function compared to patients with normal coupling (p for all < 0.001). With regards to RV free-lateral-wall strain, no significant differences between both groups could be found (− 18 vs. − 21%, p = 0.074). We were able to measure all 5 echocardiographic RV function parameters in 134 (86%) individuals. Inter-observer variability showed moderate-to-good agreement in a subset of 20 patients (ICC for all > 0.704, Supplemental Table 1). Patients with impaired RV–PA coupling had a more dilated RV (end-diastolic diameter: 39 vs. 36 mm, p < 0.001), alongside with advanced TR severity (grade ≥ II: 92 vs. 68%, p < 0.001). Concomitant T-TEER was performed in 36 (23%) patients. Baseline TAPSE/PASP ratio < 0.36 was similar among patients with and without additional T-TEER (72 vs. 62%, p = 0.247).
With regards to hemodynamic assessment, mPAP (25 vs. 19 mmHg), sPAP (37 vs. 34 mmHg), and PAWP (14 vs. 10 mmHg) were significantly higher in patients with impaired RV–PA coupling (p for all < 0.047). We found only modest correlation between conventional TAPSE/PASP ratio and invasively measured sPAP (TAPSE/sPAPinv ratio: r = 0.5, p < 0.001). Nevertheless, among patients with impaired coupling on echocardiography, TAPSE/sPAPinv ratio remained significantly lower, when compared to normal coupling (0.40 vs. 0.57, p < 0.001).
RV–PA coupling and cardiovascular outcomes
A total of 59 events (38 deaths, 21 HFH) occurred during follow-up (mean 19.5 ± 12.3 months). By Kaplan–Meier estimates, TAPSE/PASP ratio ≥ 0.36 was significantly associated with event-free survival at 2 years (log-rank: p < 0.001, Graphical Abstract). Similarly, when stratified for primary and secondary MR, impaired RV–PA coupling was found to have a significant impact on outcome (primary MR: log-rank: p = 0.009, secondary MR: log-rank: p = 0.026, Fig. 1). Cox-regression models demonstrating the association of RV–PA coupling with the primary endpoint of HFH and death are shown in Table 4 and Supplemental Table 2. After adjustment for the EuroSCORE II and NT-proBNP levels (Model B), TAPSE/PASP ratio < 0.36 on echocardiography was the only RV function parameter independently associated with outcome (adj.HR 2.65 [95% CI 1.32–5.34], p = 0.006). Findings were consistent across all multivariable models (Table 4 and Supplemental Table 2), also after excluding patients who underwent concomitant T-TEER (adj.HR 3.10 [95% CI 1.42–6.77], p = 0.004).
Reverse RV remodeling
Comprehensive follow-up data were available in 129 (83%) patients following M-TEER (mean 15.7 ± 11.2 months), of whom 28 (22%) underwent concomitant T-TEER. NYHA functional capacity significantly improved after M-TEER in both patients with impaired (NYHA grade ≥ III: 84–16%, p < 0.001) and normal RV–PA coupling at baseline (NYHA grade ≥ III: 92–19%, p < 0.001, Fig. 2A). NT-proBNP levels and Creatinine decreased significantly in individuals with impaired coupling (3537 to 2431 pg/mL, Fig. 2B, 1.3–1.2 mg/dL, p for both < 0.044), but remained unchanged in the group with preserved TAPSE/PASP ratio (2438–1805 pg/mL, 1.2–1.3 mg/dL, p for both > 0.366).
Following M-TEER, RV–PA coupling improved globally (TAPSE: ∆ + 3 mm, PASP: ∆ − 10 mmHg, p for both < 0.001, Fig. 3A), both in patients with primary MR (TAPSE: ∆ + 2 mm, PASP: ∆ − 12 mmHg, p for both < 0.001) and secondary MR (TAPSE: ∆ + 2.5 mm, PASP: ∆ − 9 mmHg, p for both < 0.001). However, changes were more profound in the group with impaired coupling (TAPSE: ∆ + 2 mm, PASP: ∆ − 13 mmHg, p for both < 0.001), when compared to normal coupling at baseline (TAPSE: ∆ + 1.5 mm, p < 0.001, PASP: ∆ − 6 mmHg, p = 0.156). Supplemental Fig. 1 shows changes in TAPSE/PASP ratio stratified for post-procedural MR. Similarly, at follow-up, RV size decreased only in patients with impaired coupling, but did not change in patients with normal coupling (RV end-diastolic diameter: ∆ − 5.5 mm vs. ∆ − 3 mm, p < 0.001 and p = 0.113, respectively). With regards to TR, following M-TEER, in both groups, a significant reduction of TR severity could be achieved (impaired vs. normal coupling, grade ≥ II: 91–69% and 67–44%, p for both < 0.027, Fig. 3B). Of note, these changes remained consistent among patients with impaired coupling at baseline and in absence of concomitant T-TEER (TR grade ≥ II: 88–65%, p = 0.002), but not in patients with preserved TAPSE/PASP ratio (TR grade ≥ II: 61–39%, p = 0.112).
Discussion
In this comprehensive analysis of M-TEER patients, we observed that (1) TAPSE/PASP ratio was the only RV function parameter significantly associated with outcome in primary as well as secondary MR; (2) reverse RV remodeling, alongside with a reduction of TR severity, was evident in the majority of individuals following M-TEER; however, (3) these changes were only significant in patients with impaired RV–PA coupling at baseline.
M-TEER has increasingly been performed in recent years; however, pre-procedural risk assessment remains challenging. So far, accepted prognostic factors for mid- and long-term prognosis include pulmonary hypertension [21], renal failure [22], poor LVEF, and post-procedural residual MR [23, 24]. Only a few studies have investigated the prognostic impact of RV function in patients undergoing M-TEER. Godino et al. [25] found that TAPSE < 16 mm and/or S’ < 10 cm/s was not associated with outcome up to 2 years after M-TEER. Conversely, registry data of 817 patients undergoing M-TEER showed that impaired RV–PA coupling, defined as TAPSE/PASP ratio ≤ 0.274, was associated with a twofold increased risk of death at 2 years. [8] In addition, Adamo et al. [26] reported that two-thirds of 501 patients improved their RV–PA coupling after M-TEER, which was also associated with better outcomes. Of note, all these studies solely included patients with secondary MR. In the present study, we were able to expand on these findings by demonstrating that TAPSE/PASP ratio on echocardiography—rather than derived from right heart catheterization or other echocardiographic RV indices—was independently associated with HFH and death, irrespective of the underlying MR etiology. Our findings are of particular interest, especially considering the recently published recommendation level IIa for M-TEER in the treatment of both primary and secondary MR [2]. Notably, the indication upgrade for primary MR is based on registry data [23] and results from randomized-controlled studies, such as the upcoming PRIMARY trial (NCT05051033), are highly anticipated to confirm or disprove present study results.
At follow-up, we observed a significant improvement in NYHA functional capacity in all patients undergoing M-TEER, irrespective of TAPSE/PASP ratio at baseline. Although rates for HFH/death at 1 year and 2 years were higher among individuals with impaired coupling (33% and 43%, respectively), when compared to normal coupling (16% and 18%, respectively), these patients show a significant improvement in RV function and size, alongside with a reduction in TR severity at follow-up, irrespective of concomitant T-TEER. Of note, these changes are in line with previously reported data [26] and remained consistent across both MR etiologies. Hence, patients with reduced TAPSE/PASP ratio at baseline show the best potential for optimization in terms of cardiac remodeling, provided that they survive the first years following M-TEER.
The present analysis has several strengths: prospectively collected data, comprehensive hemodynamic as well as RV assessment, mid- to long-term follow-up, and inclusion of primary MR. We were able to add important new information regarding the prognostic value of RV–PA coupling in significant MR and provide further insights into patient selection and reverse RV remodeling after M-TEER.
Limitations
All data refer to the experience of a single center. However, this setting ensures consistency throughout the study period, including echocardiographic and hemodynamic assessment and post-processing workflows. Even though TTE studies were performed and interpreted by different investigators, inter-observer variability showed reasonable agreement. Of note, echocardiographic evaluation of PASP in the presence of significant TR must be interpreted with caution, thus may contributing to the only modest correlation between invasive and non-invasive measurements. In addition, baseline echocardiographic and hemodynamic assessment were performed at different time points throughout the study course. Hence, a respective bias with regards to variable fluid status cannot be completely ruled out. Finally, our study was not designed to compare prognostic implications of treatment alternatives, such as surgical MV treatment or medical therapy.
Conclusion
In the present study, non-invasively assessed TAPSE/PASP ratio was the only RV function parameter independently associated with HFH and death in patients with significant MR. Individuals with impaired RV–PA coupling at baseline benefited from M-TEER, irrespective of the underlying MR etiology, especially in terms of clinical symptoms, reverse RV remodeling, and TR reduction 1 year after intervention.
Abbreviations
- HFH:
-
Heart failure hospitalization
- MR:
-
Mitral regurgitation
- PASP:
-
Pulmonary artery systolic pressure
- RV–PA:
-
Right ventricle-to-pulmonary artery
- TAPSE:
-
Tricuspid annular plane systolic excursion
- TR:
-
Tricuspid regurgitation
- M-TEER:
-
Transcatheter edge-to-edge mitral valve repair
- T-TEER:
-
Transcatheter edge-to-edge tricuspid valve repair
References
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W (2022) 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 43:561–632
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C (2020) ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020:CIR0000000000000923
Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, Apruzzese P, Fail P, Rinaldi MJ, Smalling RW, Hermiller JB, Heimansohn D, Gray WA, Grayburn PA, Mack MJ, Lim DS, Ailawadi G, Herrmann HC, Acker MA, Silvestry FE, Foster E, Wang A, Glower DD, Mauri L (2015) Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol 66:2844–2854
Mack MJ, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant BK, Grayburn PA, Rinaldi MJ, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Rogers JH, Marx SO, Cohen DJ, Weissman NJ, Stone GW (2021) 3-year outcomes of transcatheter mitral valve repair in patients with heart failure. J Am Coll Cardiol 77:1029–1040
Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, Richards AM, Arslan F, Ling LH (2017) Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail 19:1664–1671
Hsu S, Simpson CE, Houston BA, Wand A, Sato T, Kolb TM, Mathai SC, Kass DA, Hassoun PM, Damico RL, Tedford RJ (2020) Multi-beat right ventricular-arterial coupling predicts clinical worsening in pulmonary arterial hypertension. J Am Heart Assoc 9:e016031
Sultan I, Cardounel A, Abdelkarim I, Kilic A, Althouse AD, Sharbaugh MS, Gupta A, Xu J, Fukui M, Simon MA, Schindler JT, Lee JS, Gleason TG, Cavalcante JL (2019) Right ventricle to pulmonary artery coupling in patients undergoing transcatheter aortic valve implantation. Heart 105:117–121
Karam N, Stolz L, Orban M, Deseive S, Praz F, Kalbacher D, Westermann D, Braun D, Näbauer M, Neuss M, Butter C, Kassar M, Petrescu A, Pfister R, Iliadis C, Unterhuber M, Park S-D, Thiele H, Baldus S, Stephan-von-Bardeleben R, Blankenberg S, Massberg S, Windecker S, Lurz P, Hausleiter J (2021) Impact of right ventricular dysfunction on outcomes after transcatheter edge-to-edge repair for secondary mitral regurgitation. JACC Cardiovasc Imaging 14:768–778
Iung B, Armoiry X, Vahanian A, Boutitie F, Mewton N, Trochu J-N, Lefèvre T, Messika-Zeitoun D, Guerin P, Cormier B, Brochet E, Thibault H, Himbert D, Thivolet S, Leurent G, Bonnet G, Donal E, Piriou N, Piot C, Habib G, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Saint Etienne C, Leroux L, Gilard M, Samson G, Rioufol G, Maucort-Boulch D, Obadia JF (2019) Percutaneous repair or medical treatment for secondary mitral regurgitation: outcomes at 2 years. Eur J Heart Fail 21:1619–1627
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270
Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and t. J Am Soc Echocardiogr 23:685–688
Asami M, Stortecky S, Praz F, Lanz J, Raber L, Franzone A, Piccolo R, Siontis GCM, Heg D, Valgimigli M, Wenaweser P, Roost E, Windecker S, Pilgrim T (2019) Prognostic value of right ventricular dysfunction on clinical outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Imaging 12:577–587
Koschutnik M, Dannenberg V, Nitsche C, Donà C, Siller-Matula JM, Winter M-P, Andreas M, Zafar A, Bartko PE, Beitzke D, Loewe C, Aschauer S, Anvari-Pirsch A, Goliasch G, Hengstenberg C, Kammerlander AA, Mascherbauer J (2021) Right ventricular function and outcome in patients undergoing transcatheter aortic valve replacement. Eur Hear J Cardiovasc Imaging 22:1295–1303
Harjola V-P, Mebazaa A, Celutkiene J, Bettex D, Bueno H, Chioncel O, Crespo-Leiro MG, Falk V, Filippatos G, Gibbs S, Leite-Moreira A, Lassus J, Masip J, Mueller C, Mullens W, Naeije R, Nordegraaf AV, Parissis J, Riley JP, Ristic A, Rosano G, Rudiger A, Ruschitzka F, Seferovic P, Sztrymf B, Vieillard-Baron A, Yilmaz MB, Konstantinides S (2016) Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology. Eur J Heart Fail 18:226–241
Lancellotti P, Tribouilloy C, Hagendorff A, Moura L, Popescu BA, Agricola E, Monin J-L, Pierard LA, Badano L, Zamorano JL (2010) European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease). Eur J Echocardiogr 11:223–244
Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, Hagendorff A, Monin J-L, Badano L, Zamorano JL (2010) European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 11:307–332
Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endo. Eur Heart J 37:67–119
Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L (2011) Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 364:1395–1406
Santas E, De la Espriella R, Chorro FJ, Palau P, Miñana G, Heredia R, Amiguet M, Merenciano H, Sanchis J, Lupón J, Bayés-Genís A, Núñez J (2020) Right ventricular dysfunction staging system for mortality risk stratification in heart failure with preserved ejection fraction. J Clin Med 9:831
Gorter TM, van Veldhuisen DJ, Voors AA, Hummel YM, Lam CSP, Berger RMF, van Melle JP, Hoendermis ES (2018) Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs post-capillary pulmonary hypertension. Eur Hear J Cardiovasc Imaging. 19:425–432
Matsumoto T, Nakamura M, Yeow W-L, Hussaini A, Ram V, Makar M, Gurudevan SV, Trento A, Siegel RJ, Kar S (2014) Impact of pulmonary hypertension on outcomes in patients with functional mitral regurgitation undergoing percutaneous edge-to-edge repair. Am J Cardiol 114:1735–1739
Grayburn PA, Sannino A, Cohen DJ, Kar S, Lim DS, Mishell JM, Whisenant BK, Rinaldi MJ, Kapadia SR, Rajagopal V, Crowley A, Kotinkaduwa LN, Lindenfeld J, Abraham WT, Mack MJ, Stone GW (2020) Predictors of clinical response to transcatheter reduction of secondary mitral regurgitation: the COAPT trial. J Am Coll Cardiol 76:1007–1014
Sorajja P, Vemulapalli S, Feldman T, Mack M, Holmes DRJ, Stebbins A, Kar S, Thourani V, Ailawadi G (2017) Outcomes with transcatheter mitral valve repair in the united states: an STS/ACC TVT Registry report. J Am Coll Cardiol 70:2315–2327
Koschutnik M, Dannenberg V, Donà C, Nitsche C, Kammerlander AA, Koschatko S, Zimpfer D, Hülsmann M, Aschauer S, Schneider M, Bartko PE, Goliasch G, Hengstenberg C, Mascherbauer J (2022) Transcatheter versus surgical valve repair in patients with severe mitral regurgitation. J Pers Med. 12:90
Godino C, Salerno A, Cera M, Agricola E, Fragasso G, Rosa I, Oppizzi M, Monello A, Scotti A, Magni V, Montorfano M, Cappelletti A, Margonato A, Colombo A (2016) Impact and evolution of right ventricular dysfunction after successful MitraClip implantation in patients with functional mitral regurgitation. Int J Cardiol Hear Vasc 11:90–98
Adamo M, Inciardi RM, Tomasoni D, Dallapellegrina L, Estévez-Loureiro R, Stolfo D, Lupi L, Pancaldi E, Popolo Rubbio A, Giannini C, Benito-González T, Fernández-Vázquez F, Caneiro-Queija B, Godino C, Munafò A, Pascual I, Avanzas P, Frea S, Boretto P, Moñivas Palomero V, Del Trigo M, Biagini E, Berardini A, Nombela-Franco L, Jimenez-Quevedo P, Lipsic E, Saia F, Petronio AS, Bedogni F, Sinagra G, Guazzi M, Voors A, Metra M (2022) Changes in right ventricular-to-pulmonary artery coupling after transcatheter edge-to-edge repair in secondary mitral regurgitation. JACC Cardiovasc Imaging 15:2038–2047
Funding
Open access funding provided by Medical University of Vienna. The authors received no specific funding for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
V. Dannenberg received consulting fees from Abbott, and educational grants from Edwards Lifesciences. J. Mascherbauer received proctor fees from Abbott and Edwards Lifesciences, consulting fees from Boston Scientific, Edwards Lifesciences, and Shockwave Medical, and educational grants from Abbott, Boston Scientific, and Edwards Lifesciences. The remaining authors have nothing to disclose.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koschutnik, M., Donà, C., Nitsche, C. et al. Impact of right ventricular-to-pulmonary artery coupling on remodeling and outcome in patients undergoing transcatheter edge-to-edge mitral valve repair. Clin Res Cardiol (2023). https://doi.org/10.1007/s00392-023-02318-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00392-023-02318-w