Abstract
Background
Percutaneous coronary intervention (PCI) is standard of care in patients with acute coronary syndrome (ACS) suitable for interventional revascularization. Intracoronary imaging by optical coherence tomography (OCT) expanded treatment approaches adding diagnostic information and contributing to stent optimization.
Objectives
This meta-analysis aimed to assess the effects of OCT-guided vs. angiography-guided PCI in treatment of ACS.
Methods
A structured literature search was performed. All controlled trials evaluating OCT-guided vs. angiography-guided PCI in patients with ACS were eligible. The primary end point was major adverse cardiac events (MACE).
Results
Eight studies enrolling 2612 patients with ACS were eligible. 1263 patients underwent OCT-guided and 1,349 patients angiography-guided PCI. OCT guidance was associated with a 30% lower likelihood of MACE (OR 0.70, 95% CI 0.53–0.93, p = 0.01, I2 = 1%). OCT-guided PCI was also associated with significantly decreased cardiac mortality (OR 0.49, 95% CI 0.25–0.96, p = 0.04, I2 = 0%). There was no detectable difference in all-cause mortality (OR 1.08, 95% CI 0.51–2.31, p = 0.83, I2 = 0). Patients in OCT-guided group less frequently required target lesion revascularization (OR 0.26, 95% CI 0.07–0.95, p = 0.04, I2 = 0%). Analysis of myocardial infarction did not result in significant treatment differences. In subgroup or sensitivity analysis the observed advantages of OCT-guided PCI were not replicable.
Conclusion
The evidence suggests that PCI guidance with OCT in ACS decreases MACE, cardiac death and target lesion revascularization compared to angiography. On individual study level, in subgroup or sensitivity analyses these advantages were not thoroughly replicable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute coronary syndrome (ACS) is a life-threatening disease with high morbidity and mortality burden. Immediate patient management is required, and invasive coronary angiography remains the diagnostic standard [1, 2]. Coronary angiography allows adequate treatment by percutaneous coronary intervention (PCI) [1, 2]. Balloon angioplasty followed by stent implantation is the predominant revascularization strategy, but in selected cases balloon angioplasty only, thrombus aspiration or conservative management is preferred over stent deployment [1, 3,4,5]. For decades, stent implantation was based only on fluoroscopic findings [6]. Quantitative standards and measurements were limited until recent technical developments in intracoronary imaging or functional assessment expanded the interventional repertoire [6]. Imaging-guided PCI with stent implantation in an elective setting was advantageous compared to conventional angiography resulting in a significant reduction of cardiac death and major adverse cardiac events previously [7]. Optical coherence tomography (OCT) and intravascular ultrasonography (IVUS) are the most frequently used imaging techniques [6, 8].
OCT is a light-based imaging technique in near-infrared spectrum with excellent near-field imaging, but technically axial range is limited and use depends on blushing the examined artery [9,10,11]. OCT allows detection of the underlying pathology of ACS, for example, plaque rupture can be distinguished from plaque erosion [10]. Precise measurements and visualization enable defining landing zone or optimal sizing in infarct-related arteries. After stent deployment, OCT may diagnose edge dissection, stent underexpansion, malapposition, or residual disease [10]. The applicability of OCT in PCI was demonstrated in elective circumstances before, but data on efficacy under urgent conditions during ACS are limited [7, 12, 13]. The current meta-analysis aimed to comprehensively assess the effects of OCT-guided compared to angiography-guided PCI in ACS based on efficacy outcomes.
Methods
This meta-analysis was conducted using a pre-specified protocol and reproducible plan for literature search and synthesis according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines [14].
Controlled trials comparing OCT-guided to angiography-guided PCI in patients with ACS requiring interventional revascularization were included. Randomized controlled trials (RCT) and non-randomized controlled studies (NRS) were eligible. All details regarding search strategy, data extraction, and study selection are in presented in suppl. material 1.
The primary end point was major adverse cardiac events (MACE), a composite of cardiac mortality, myocardial infarction, and target vessel revascularization (TVR). The individual components of MACE, all-cause mortality, and target lesion revascularization (TLR) were secondary efficacy endpoints.
Risk of bias at study level was assessed using the Cochrane Collaborations risk of bias tool (RoB2, version 08/22/2019) for randomized trials [15]. Non-randomized controlled studies were assessed using the Cochrane Collaborations Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I, version 10/20/2016) tool [16]. Risk of bias assessment was performed by two individual investigators (SMM, SH). In case of discrepancy a third independent investigator was consulted (MMM).
Random-effects meta-analyses were performed using the Mantel–Haenszel method for dichotomous data. Pooled odds ratios (ORs) and 95% confidence intervals (CI) are given for each analysis with a two-sided significance level of p < 0.05 (RevMan 5.3, Nordic Cochrane Centre, Cochrane Collaboration). The extent of heterogeneity was approximated by I2 tests considering 0–40% as non-important, 30–60% as moderate, 50–90% as substantial, and 75–100% as considerable heterogeneity. Pre-specified analysis of publication bias by funnel plot was not appropriately feasible given the low number of studies included.
Follow-up varied between the trials and timing of measurement of outcomes during the study period was heterogeneously performed. Consequently, ORs were calculated from event data of longest follow-up of each study. Post hoc subgroup analyses were performed to test whether timing of OCT (OCT pre- or post-stent implantation) affected the results. This approach allowed differentiation between the use of OCT as diagnostic tool compared to stent optimization and subsequent therapeutically consequences.
Post hoc sensitivity meta-analyses were performed according to risk of bias judgement to assess the impact of study quality on the investigated outcomes. RCTs with “high” risk of overall bias were excluded from sensitivity analysis. NRS with “serious” or “critical” risk of overall bias were eliminated from sensitivity analyses.
We did not obtain ethical approval for this meta-analysis because we did not collect data from individual human subjects.
Results
Study selection
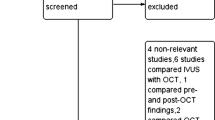
A total of 1706 articles were identified by the described search strategy (see Fig. 1, PRISMA Flow chart). After removing duplicates, titles and abstracts of 1501 remaining articles were screened. 1446 articles were excluded which left 55 references for assessment of the full-text articles. Six additional full texts were assessed for eligibility by handsearching. Considering exclusion criteria eight studies were finally included in quantitative analyses [17,18,19,20,21,22,23,24].
Studies
Eight controlled studies were included in meta-analysis [17,18,19,20,21,22,23,24] (see Table 1). Of these, three were RCTs [18, 23, 24]. Four trials were conducted as case series studies with propensity score matching (PSM), notably D’Ascenzo et al. and Iannaccone et al. each conducted PSM pooling data from the OCT-FORMIDABLE registry [17, 19,20,21]. The lasting study was designed as case series study without matching [22]. The use of OCT varied in the trials: three studies used OCT preliminary to PCI as diagnostic tool [20,21,22], one trial investigated stent optimization and used OCT solely after PCI [17], and four studies used OCT both in the pre- and post-stent implantation period [18, 19, 23, 24].
Assessment of bias
Assessment of bias was performed in three RCTs using RoB2 tool [18, 23, 24]. Two RCTs were associated with “some concerns” in overall judgement (see Table 2). The OCTACS trial was judged to be at “high” risk of overall bias [24]. This judgement was mainly caused by substantial deviations from defined intervention because two patients underwent imaging using IVUS instead of OCT and one patient with peri-interventional complication likely caused by OCT was excluded from efficacy analysis.
Potential sources of bias were assessed in five NRS by ROBINS-I tool [17, 19,20,21,22]. Three trials were judged to have moderate risk of overall bias, whereas two trials were considered to have critical risk of overall bias (see Table 3). The latter were included in the overall analysis, but were precluded from sensitivity analysis. The overall judgement “critical” risk of bias was based on severe differences in patient selection and varying treatment periods and protocols between OCT and comparator group [17, 22].
Patient-level baseline characteristics and procedural data
A total of 2612 patients with ACS were included. Baseline characteristics are summarized in Table 1. The median age ranged from 54.5 to 73 years, and 78.3% were male. Analysis of cardiovascular risk profile revealed that 22.7% had diabetes, 58.2% had dyslipidemia. 63.7% had hypertension, and 49% were ever smokers. STEMI was the predominant type of ACS, followed by NSTEMI and unstable angina. All patients were managed invasively. 1263 patients underwent OCT-guided and 1349 patients angiography-guided PCI. Stent implantation was the predominant revascularization strategy. The follow-up period ranged from 6 to 25 months.
Primary outcome analysis
Seven trials reporting on 1141 patients treated with OCT-guided PCI compared to 1230 patients undergoing angiography-guided PCI were included in quantitative analysis of major adverse cardiac events [17,18,19,20,21,22, 24]. EROSION III did not explicitly report TVR and was excluded from primary outcome analysis [23]. MACE rate was 8.7% in OCT-guided PCI group compared to 12.3% in angiography-guided group. OCT guidance led to a significant difference with a 30% lower likelihood of MACE (OR 0.70, 95% CI 0.53–0.93, p = 0.01, I2 = 1%, non-relevant heterogeneity, see Fig. 2).
Secondary outcome analyses
Overall, eight trials were included in secondary outcome analyses [17,18,19,20,21,22,23,24].
All-cause mortality
Five trials reporting on 667 patients treated with OCT-guided PCI compared to 672 patients treated with angiography-guided PCI were analyzed [17, 18, 20, 21, 24]. All-cause mortality rate was 2.4% in OCT-guided PCI group compared to 2.1% in angiography-guided group without a statistical difference (OR 1.08, 95% CI 0.51–2.31, p = 0.83, I2 = 0%, non-relevant heterogeneity, see Fig. 3a).
a Secondary analyses: all-cause mortality. b Secondary analyses: cardiac mortality. c Secondary analyses: myocardial infarction. d Secondary analyses: target vessel revascularization. e Secondary analyses: target lesion revascularization. OCT optical coherence tomography, PCI percutaneous coronary intervention, M-H Mantel–Haenszel method, CI confidence interval
Cardiac mortality
Four trials reporting on 626 patients treated with OCT-guided PCI compared to 717 patients undergoing angiography-guided PCI were included [19, 22,23,24]. Cardiac mortality rate was 2.2% in OCT-guided PCI group compared to 4.0% in angiography-guided group. OCT-guided PCI led to a significant difference with a 51% lower likelihood of cardiac mortality (OR 0.49, 95% CI 0.25–0.96, p = 0.04, I2 = 0%, non-relevant heterogeneity, see Fig. 3b).
Myocardial infarction
Seven trials reporting on 1056 patients treated with OCT-guided PCI compared to 1147 patients undergoing angiography-guided PCI were analyzed [17,18,19, 21,22,23,24]. Myocardial infarction rate was 2.6% in OCT-guided PCI group compared to 3.2% in angiography-guided group without a statistical difference (OR 0.82, 95% CI 0.49–1.37, p = 0.45, I2 = 0%, non-relevant heterogeneity, see Fig. 3c).
Target vessel revascularization
Seven trials reporting on 1213 patients treated with OCT-guided PCI compared to 1304 patients undergoing angiography-guided PCI were included [18,19,20,21,22,23,24]. TVR rate was 2.4% in OCT-guided PCI group compared to 5.4% in angiography-guided group without a statistical difference (OR 0.54, 95% CI 0.26–1.13, p = 0.10, I2 = 45%, moderate heterogeneity, see Fig. 3d).
Target lesion revascularization
Three trials reporting on 570 patients treated with OCT-guided PCI compared to 445 patients undergoing angiography-guided PCI were analyzed [21, 22, 24]. TLR rate was 0.5% in OCT-guided PCI group compared to 2.7% in angiography-guided group. OCT-guided PCI led to a significant difference with a 74% lower likelihood of TLR (OR 0.26, 95% CI 0.07–0.95, p = 0.04, I2 = 0%, non-relevant heterogeneity, see Fig. 3e).
Subgroup and sensitivity analyses did not demonstrate any statistically significant difference in primary or secondary outcomes (see Suppl. Table 1, Suppl. Table 2, and Suppl. Material 1).
Discussion
To our knowledge, this is the first meta-analysis comprehensively assessing the effect of OCT-guided compared to angiography-guided PCI in patients with ACS. The main and novel findings of pooled data analyses were as follows:
-
OCT-guided PCI resulted in a 30% lower likelihood of MACE and 74% lower odds of target lesion revascularization,
-
OCT-guided PCI led to significant decrease of 51% in cardiac mortality, but this did not translate to difference in all-cause mortality.
Pooled data analysis is the uncontested strength of meta-analysis, but interpretation of overall effects requires cautious revision. On individual study level only Khalifa et al. demonstrated a significant MACE reduction and Iannaccone et al. exclusively reported a directed OR favoring OCT in TLR [21, 22]. All other trials did not show a significantly directed treatment effect according to MACE, cardiac death, or TLR on individual study level (see Figs. 2 and 3a–e). Moreover, in subgroup or sensitivity analyses the observed efficacy advantages were not thoroughly replicable. Study heterogeneity, study quality and low event rates might be possible explanations.
Then, pooled data analysis resulted in a significantly decreased cardiac mortality in OCT group, but all-cause mortality (OR 1.08, 95% CI 0.51–2.31) did not differ in overall analysis. Noteworthy, only OCTACS trial was eligible for both mortality analyses and this study itself lacked statistical power in mortality measurement [24]. Hence, one might speculate that structural interstudy heterogeneity rather than intervention itself might have contributed to the measured effects of all-cause or cardiac mortality. Important structural differences need to be acknowledged.
In included trials OCT was mostly used to examine deployed stents and assess success of revascularization [17,18,19, 23, 24]. One might hypothesize that the treatment benefit of OCT is mostly caused by its impact on optimizing revascularization strategy rather than its diagnostic role. In DOCTORS trial OCT imaging resulted in decision for stent optimization in 50% of patients and in 27% additional stents were implanted [18]. These data are in line with OCTACS and TOTAL each reporting 46% stent optimization rate following OCT [19, 24]. On the contrary, angiography led to further treatment in 22.5% of patients and additional stents were used in 18% [18]. However, the current study-level data set did not allow adequately powered subgroup calculation according to pre- or post-implantation OCT run (see suppl. Table 1). Consequently, this hypothesis cannot be validated. Potentially, a patient-level approach might add evidence whether the observed treatment effects of OCT are caused by stent optimization or whether competing mechanisms contribute to observed advantages.
Study design, performance, and study quality restrict generalizability to daily routine. Firstly, OCT was mainly performed by experienced operators and teams in designed trials. Secondly, OCT usage significantly increased procedural time (+ 13 min OCTACS, + 14 min EROSION, + 20 min TOTAL, + 20 min DOCTORS) and resulted in increased amount of contrast medium [18, 19, 23, 24]. Time delay might be harmful in urgent ACS scenario and higher contrast medium volume might decline kidney function [25]. The increased procedural duration and amount of contrast medium performing OCT was observed in elective setting likewise [13]. The EROSION III trial was intentionally designed to reduce stent implantation rate—86% of patients with plaque erosion were managed without stent implantation [23]. However, the majority of trials a priori used stenting as primary strategy to manage revascularization and subsequently used OCT not as diagnostic, but as implicit treatment tool.
The pooled meta-analysis pointed out weaknesses and gaps in the evidence. Upcoming controlled trials will not enroll all entities of ACS patients. They will not add substantial evidence to answer the current research question [26,27,28]. One might even speculate whether a RCT concomitantly enrolling STEMI and NSTEMI is reasonable given the fact that STEMI almost always requires immediate or rescue PCI [1].
Recently, implementation of the upcoming TACTICS registry was published [29]. This observational uncontrolled study will add further information on feasibility and diagnostic benefit of OCT-guided PCI in ACS patients, but underlies the limitations of a non-randomized study [29].
To definitely answer the research question future controlled studies are required. These trials should acknowledge the following terms to overcome the substantial bias domains:
-
Comparable pretreatment
-
Directed use of OCT, defined number of runs (if multiple runs are necessary), and limited operator’s discretion on treatment
-
Precisely defined stenting conditions (Indications for stent deployment? How to treat complex lesions, calcified lesions or stenotic bifurcation? Does plaque erosion require stenting?)
In the absence of adequately powered, high-quality trials the use of OCT in ACS patients undergoing PCI revascularization cannot be generally recommended. Intracoronary imaging is useful, but implementation should not withhold restoration of coronary flow. An increase of procedural time and subsequent prolonged ischemia with loss of myocardium should not outweigh the potential benefits. Timing, the operator’s experience in imaging and interpretation, local resources, and patient’s characteristics like hemodynamic stability or renal function should be considered in decision making for OCT use in ACS.
Limitations
Despite the methodological PRISMA approach, the current meta-analysis underlies inherent limitations. Specific aspects limiting generalizability should be acknowledged: we did not have access to patient-level data. Analysis of patient-level data would potentially produce more reasonable quantitative results, but was not applicable with limited access to only published data.
Patients were predominantly male and cardiovascular risk factors were unequally distributed. Two studies exclusively included STEMI patients, whereas two trials only enrolled NSTE-ACS patients. All studies were unblinded trials, and five were NRS. These facts might have contributed to selection, detection, and performance bias.
The follow-up duration and the timing of OCT runs varied between the trials. Three trials only investigated OCT use prior to stent implantation, whereas all other trials examined stent optimization strategy by OCT. Two trials did not report on stenting strategy and the EROSION III trial was conducted to reduce stent implantation rate by intracoronary imaging. These aspects indicate substantial performance bias.
The judgement of risk of bias on trial level demonstrated variance in study quality. This heterogeneity resulted in the authors’ decision to add further post hoc sensitivity analysis. The current meta-analysis only measured efficacy end points, safety outcomes like bleeding, or vascular access complication were not assessed.
Conclusion
The evidence suggests that PCI guidance with OCT in ACS decreases MACE, cardiac death, and target lesion revascularization compared to angiography in pooled data analysis. On individual study level, in subgroup or sensitivity analyses these advantages were not thoroughly replicable. Study heterogeneity, study quality, and low event rates might be possible explanations.
Future randomized clinical trials with adequate statistical power and enrollment of all ACS entities are required to clarify the role of OCT as stent optimization in ACS revascularization procedures.
Abbreviations
- ACS:
-
Acute coronary syndrome
- CI:
-
Confidence interval
- FFR:
-
Fractional flow reserve
- IVUS:
-
Intravascular ultrasonography
- MACE:
-
Major adverse cardiac event
- NRS:
-
Non-randomized controlled studies
- NSTE-ACS:
-
Non-ST segment elevation acute coronary syndrome
- NSTEMI:
-
Non-ST segment elevation myocardial infarction
- OCT:
-
Optical coherence tomography
- OFDI:
-
Optical frequency domain imaging
- OR:
-
Odds ratio
- PCI:
-
Percutaneous coronary intervention
- PSM:
-
Propensity score matching
- RCT:
-
Randomized controlled trial
- STEMI:
-
ST segment elevation myocardial infarction
- TLR:
-
Target lesion revascularization
- TVR:
-
Target vessel revascularization
References
Ibanez B, James S, Agewall S et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC). Eur Heart J 39:119–177
Collet JP, Thiele H, Barbato E et al (2021) 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 42:1289–1367
Neumann FJ, Chettibi M, Sisakia H et al (2019) 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 40:87
Jolly SS, Cairns JA, Yusuf S et al (2016) Outcomes after thrombus aspiration for ST elevation myocardial infarction: 1-year follow-up of the prospective randomised TOTAL trial. Lancet 387:127–135
Jolly SS, James S, Dzavik V et al (2017) Thrombus aspiration in ST-segment-elevation myocardial infarction: an individual patient meta-analysis: thrombectomy trialists collaboration. Circulation 135:143–152
Mintz GS, Guagliumi G (2017) Intravascular imaging in coronary artery disease. Lancet 390:793–809
Buccheri S, Franchina G, Romano S et al (2017) Clinical outcomes following intravascular imaging-guided versus coronary angiography-guided percutaneous coronary intervention with stent implantation: a systematic review and bayesian network meta-analysis of 31 studies and 17,882 patients. JACC Cardiovasc Interv 10:2488–2498
Wang J, Yuan S, Qi JJ, Zhang QG, Ji Z (2022) Advantages and prospects of optical coherence tomography in interventional therapy of coronary heart disease (review). Exp Ther Med 23:19
Huang DSE, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA et al (1991) Optical coherence tomography. Science 254:1178–1181
Araki M, Park SJ, Dauerman HL et al (2022) Optical coherence tomography in coronary atherosclerosis assessment and intervention. Nat Rev Cardiol 19:684–703
Nagaraja V, Kalra A, Puri R (2020) When to use intravascular ultrasound or optical coherence tomography during percutaneous coronary intervention? Cardiovasc Diagn Ther 10:1429–1444
Kubo T, Shinke T, Okamura T et al (2017) Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J 38:3139–3147
Ali ZA, Maehara A, Genereux P et al (2016) Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet 388:2618–2628
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 10:89
Sterne JAC, Savovic J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898
Sterne JA, Hernan MA, Reeves BC et al (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
Di Giorgio A, Capodanno D, Ramazzotti V et al (2013) Optical coherence tomography guided in-stent thrombus removal in patients with acute coronary syndromes. Int J Cardiovasc Imaging 29:989–996
Meneveau N, Souteyrand G, Motreff P et al (2016) Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome results of the multicenter, randomized DOCTORS study (does optical coherence tomography optimize results of stenting). Circulation 134:906
Sheth TN, Kajander OA, Lavi S et al (2016) Optical coherence tomography-guided percutaneous coronary intervention in ST-segment-elevation myocardial infarction: a prospective propensity-matched cohort of the thrombectomy versus percutaneous coronary intervention alone trial. Circ Cardiovasc Interv 9:e003414
D’Ascenzo F, Iannaccone M, De Filippo O et al (2017) Optical coherence tomography compared with fractional flow reserve guided approach in acute coronary syndromes: a propensity matched analysis. Int J Cardiol 244:54–58
Iannaccone M, D’Ascenzo F, Frangieh AH et al (2017) Impact of an optical coherence tomography guided approach in acute coronary syndromes: a propensity matched analysis from the international FORMIDABLE-CARDIOGROUP IV and USZ registry. Catheter Cardiovasc Interv 90:E46-e52
Khalifa AKM, Kubo T, Shimamura K et al (2021) Impact of optical coherence tomography imaging on decision-making during percutaneous coronary intervention in patients presented with acute coronary syndromes. Circ J 85:1781–1788
Jia HB, Dai JN, He LP et al (2022) EROSION III a multicenter RCT of OCT-guided reperfusion in STEMI with early infarct artery patency. JACC Cardiovasc Interv 15:846–856
Antonsen L, Thayssen P, Maehara A et al (2015) Optical coherence tomography guided percutaneous coronary intervention with nobori stent implantation in patients with non-ST-segment-elevation myocardial infarction (OCTACS) trial: difference in strut coverage and dynamic malapposition patterns at 6 months. Circ Cardiovasc Interv 8:e002446
Nemoto T, Minami Y, Sato T et al (2019) Contrast volume and decline in kidney function in optical coherence tomography-guided percutaneous coronary intervention. Int Heart J 60:1022–1029
Kubo T, Shinke T, Okamura T et al (2018) Comparison between Optical COherence tomography guidance and Angiography guidance in percutaneous coronary intervention (COCOA): study protocol for a randomized controlled trial. J Cardiol 72:170–175
Holm NR, Andreasen LN, Walsh S et al (2018) Rational and design of the European randomized optical coherence tomography optimized bifurcation event reduction trial (OCTOBER). Am Heart J 205:97–109
Ali Z, Landmesser U, Karimi Galougahi K et al (2021) Optical coherence tomography-guided coronary stent implantation compared to angiography: a multicentre randomised trial in PCI—design and rationale of ILUMIEN IV: OPTIMAL PCI. EuroIntervention 16:1092–1099
Yamamoto MH, Kondo S, Mizukami T et al (2022) Rationale and design of the TACTICS registry: optical coherence tomography guided primary percutaneous coronary intervention for patients with acute coronary syndrome. J Cardiol 80:505–510
Funding
Open Access funding enabled and organized by Projekt DEAL. None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SMM, MMM, SH, SB, TT, HW, VM, SB, CA, SL: None.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Macherey-Meyer, S., Meertens, M.M., Heyne, S. et al. Optical coherence tomography-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndrome: a meta-analysis. Clin Res Cardiol 113, 967–976 (2024). https://doi.org/10.1007/s00392-023-02272-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02272-7