Abstract
Background
Recent data demonstrated the benefit of left atrial appendage (LAA)-amputation in patients with atrial fibrillation (AF). However, the long-term impact of LAA-amputation for patients with new-onset perioperative atrial fibrillation (POAF) is still unknown.
Methods
Patients with no history of AF undergoing coronary artery bypass grafting by off-pump technique (OPCAB) between 2014 and 2016 were retrospectively examined. Cohorts were divided by the concomitant execution of LAA-amputation. Propensity score (PS) matching was applied by all available baseline characteristics. The composite of all-cause mortality, stroke and rehospitalization in patients with POAF and patients maintaining sinus rhythm posed as the primary endpoint.
Results
A total of 1522 patients were enrolled, of whom 1208 and 243 were included in the control and the LAA-amputation group, respectively and were matched to 243 patients in each group. In total, patients with POAF without LAA-amputation showed a significantly higher rate of the composite endpoint (17.3% vs 32.1%, p = 0.007). However, patients with LAA-amputation showed no significant difference in the composite endpoint (23.2% vs 26.7%, p = 0.57). The significantly higher occurrence of the composite endpoint was driven by all-cause mortality (p = 0.005) and rehospitalization (p = 0.029). Subgroup analysis revealed a CHA2DS2-VASc-score of ≥ 3 to be associated with the high rate of the primary endpoint (p = 0.004).
Conclusion
POAF is associated with a higher rate of the combined endpoint of all-cause mortality, stroke and rehospitalization. The composite endpoint in patients with LAA-amputation concomitant with OPCAB surgery developing new-onset POAF in a 5-year follow-up was not increased compared to a control cohort maintaining sinus rhythm.
Graphical abstract
Five-year outcome of patients with POAF and LAA-amputation; 95% CI, 95% confidence interval, CPR, cardiopulmonary resuscitation, ECLS, extracorporeal life support, HR, hazard ratio, IABP, intra-aortic balloon pump, LAA, left atrial appendage, OPCAB, off-pump coronary artery bypass grafting, PAPs, systolic pulmonary artery pressure, SR, sinus rhythm, VT, ventricular tachycardia.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perioperative atrial fibrillation (POAF) is a common complication in cardiac surgery, associated with a high rate of long-term atrial fibrillation (AF), decreased cognitive and renal function, as well as an elevated use of medical resources such as extended hospitalizations and the need of intensive care and also an increased mortality rate [1,2,3]. The occurrence rate of POAF is varying between 20 and 50% [4, 5]. Therefore, the current guidelines recommend restoring sinus rhythm by antiarrhythmic medication, as well as electrical cardioversion, and reducing the risk of thromboembolic stroke by anticoagulation [6].
Once POAF occurs, the range of complications is similar to none-surgery related atrial fibrillation. For that reason, surgery-related AF, along with several other factors predisposing to develop AF such as age, genetic predisposition, diabetes mellitus, arterial hypertension, or obesity [7,8,9], should be considered as a relevant comorbidity (Fig. 1).
The baseline strategy and gold standard for stroke prevention in persistent AF is medical anticoagulation [6]. Since the left atrial appendage (LAA) is known to be the major origin of stroke caused by thrombi [10], the exclusion of the left atrial appendage by intervention or surgery (concomitant with cardiac surgery) has become a field of rising interest [10].
In fact, the LAA is easy to access during cardiac surgery by median sternotomy or anterolateral thoracotomy, leading to develop and establish techniques to exclude the LAA through ligation or amputation concomitant with e.g. coronary or valvular cardiac surgery [11, 12]. The previous hesitant recommendations to perform LAA-amputation have been strengthened by increasing evidence of stroke prevention through LAA-amputation in patients with AF, with the most famous to mention LAAOS III trial [13].
However, stroke prevention through LAA-amputation has also been observed in patients with sinus rhythm and a high CHA2DS2-VASc-score [14]. Thus, the prophylactic impact of LAA-amputation on stroke, all-cause mortality, and rehospitalization might be most effective in POAF patients. However, a dedicated analysis with long-term follow-up in POAF patients undergoing LAA-amputation has yet to be performed.
Patients and methods
Ethical statement
Approval including patient consent waiver was obtained by the local ethics committee of the Ruhr University Bochum (No: 2020-688_1; Date: 12.08.2022). Every patient received a thoroughly dedicated patient information concerning the LAA-amputation. The investigation was performed in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (www.strobe-statement.org). Furthermore, the study was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Patient recruitment and follow up
All patients undergoing isolated coronary artery bypass grafting in off-pump technique (OPCAB) between January 2014 and December 2016 in our department (Herz- und Diabeteszentrum NRW, Bad Oeynhausen, Germany) were retrospectively selected in a single-center assessment. Preoperative exclusion criteria were a history of AF, valvulopathy, and pulmonary arterial hypertension. Perioperative exclusion criteria were the need of a permanent pacemaker, extracorporeal life support, intraaortic balloon pump, ventricular tachycardia, resuscitation, or defibrillation. A detailed description of the patient recruitment strategy was given before [15].
Surgical technique
The surgical procedure was performed utilizing the off-pump technique through median sternotomy, and well-established grafts such as the left and/or right internal mammary artery, radial artery, and saphenous vein were employed. After pericardial exposure the accessibility of the LAA was judged by the surgeon. If the LAA was deemed accessible and suitable for amputation, the procedure was completed by ligation, LAA resection, and double continuous suture. Transesophageal echocardiography was conducted both before and after LAA amputation to detect any pre-existing thrombus formation or residual appendage post-amputation.
Anticoagulation
All patients were initially treated with dual antiplatelet therapy consisting of acetylsalicylic acid 100 mg and Clopidogrel 75 mg (a P2Y12 inhibitor). The dual antiplatelet regimen was recommended for a duration of 6 months, after which a transition to mono antiplatelet therapy with acetylsalicylic acid 100 mg was advised. If POAF occurred, a switch of the dual antiplatelet therapy to a combination of single antiplatelet (acetylsalicylic acid 100 mg) and oral anticoagulation strategy (Phenprocoumon) for at least 3 months was recommended. Further follow-up of the anticoagulation therapy was not part of the presented data.
POAF
To retrospectively detect the occurrence of POAF, a detailed browsing flowchart was developed, as described before [15]. In brief, prehospital reports, in-hospital transfer and discharge reports, any custodial and visit documentation in the data management systems of the intensive (COPRA, COPRA System GmbH; Germany) and standard care (ORBIS, Dedalus HealthCare, Germany) units were analyzed for any signs of arrhythmia. Furthermore, patient data was inspected for the use of antiarrhythmic medication, whereas a rhythm control was achieved by per standard used postoperative telemetry until discharge. ECG was performed at least four times for each patient (on admission, immediately after surgical intervention, first postoperative day, and before discharge). A flowchart summarizing the POAF detection protocol is provided in Fig. 2.
Outcome
The primary outcome was defined as a composite endpoint of all-cause mortality, stroke and rehospitalization. Secondary endpoints were defined as the isolated parameters of the composite endpoint. The endpoint stroke was defined as either the confirmation of stroke through cerebral imaging (computed tomography or MRI) or the presence of unequivocal neurological impairment, such as hemiplegia. Additionally, instances of stroke confirmed by a neurologist reported to our department during the follow-up period were also included in the definition. The endpoints were assessed by using four sources of information: a review of our medical records; an annual, standardized form (post-discharge) completed by the patients themselves, as well as by their out-patient care physician, and annual consultation with the respective registration office in case of missing post-discharge forms. Follow-up data was then assessed between the date of surgery (2014–2016) and December 2021. Patients were censored at their last follow-up.
To assess subgroup differences, subgroup analyses of older patients (age > 70) and patients with an increased stroke risk (CHA2DS2-VASc-score ≥ 3) were performed. All analyses were done in the total cohort, the LAA-amputation cohort, and the control cohort.
Statistical analysis
Statistical analysis was performed using the SPSS-Software (Version 28, IBM, New York, NY, USA) and R (Version 4.2.2, R Core Team, Vienna, Austria). Categorical variables are given as absolute and relative frequencies, continuous variables as means with standard deviations. Since the surgical technique was chosen in a nonrandomized fashion, we used 1:1 PS matching by 20 baseline characteristics using nearest neighbor matching with a caliper of 0.2 (Table 1), which was described in detail before[15], resulting in groups of 243 patients with and without LAA-amputation. To assess the patient outcome, patients were divided into an SR group and a POAF group. To conclude, the balance of baseline covariates was assessed by computing the z-difference (balance achieved, if ≤ |± 1.96|) and for categorical variables by additionally computing the standardized mean difference (balance achieved if < 0.1) [16, 17].
A time-to-event analysis was performed regarding the primary composite endpoint and the secondary endpoints with the use of Kaplan–Meier survival curves, stratified log-rank testing, and a stratified Cox proportional-hazards model. Categorical baseline variables were compared with the use of the Chi-square test or Fisher’s exact test.
Parameter estimates are given with their hazard ratio, 95% confidence interval (95% CI) and the corresponding p value. p values < 0.05 were considered statistically significant.
Results
Cohort
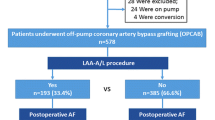
After the exclusion of 59 patients (4.7%) in the control group and 12 patients (4.7%) in the LAA-amputation group due to perioperative exclusion criteria, a total of 1208 and 243 patients were included, respectively. These were paired via PS matching to 243 patients in each group, achieving a standardized mean difference of < 0.1 and a z-difference of ≤ |± 1.96|. Twenty (8.2%) of the patients in the LAA group were included despite showing a CHA2DS2VASc-score of 1, due to smoke/thrombus formation in perioperative transesophageal echocardiography. The mean age of the patients was 69.6 (8.1) and 69.1 (7.6) years in the control and LAA-amputation group, respectively, while 19.0% and 21.4% were female. POAF after OPCAB was observed in 31.2% of LAA patients compared to 33.3% in the control group (HR 0.94; 95% CI [0.62; 1.41], p = 0.75) [15]. The baseline characteristics of the matched cohorts are illustrated in Table 1. Baseline characteristics divided by the development of POAF are provided in supplementary table 1.
POAF in the total cohort
Five-year results revealed a significantly higher frequency of the composite endpoint in the POAF group with 20.3% vs. 29.5% (HR 1.5 95% CI [1.03; 2.19] p = 0.034) and a higher all-cause mortality 11.2% vs. 20.5% (HR 1.8 95% CI [1.14; 2.95] p = 0.012) (Table 2, Fig. 3A).
Subgroup analysis in the total cohort
In the subgroup analysis of the total cohort regarding elderly patients (age > 70), a tendency towards a higher occurrence of the composite endpoint was observed [22.2% vs 31.1 (HR 1.5 95% CI [0.94; 2.42]) p = 0.09] driven by a significantly higher rate of all-cause mortality [14.6% vs 25.2% (HR 1.8 95% CI [1.06; 3.18]) p = 0.031] and a higher susceptibility for rehospitalization [2.9 vs 7.8 (HR 2.8 95% CI [0.91; 8.55]) p = 0.07], whereas the rate of stroke did not differ (p = 0.77). In younger patients (age ≤ 70) neither the composite [18.2% vs 26.4% (HR 1.6 95% CI [0.81; 3.00]) p = 0.18] nor the single endpoints showed a significant difference.
Regarding the comorbidity dependent stroke risk, the subgroup with a CHA2DS2VASc-score < 3 did not show a notable difference of the endpoints [composite endpoint 12.4% vs 14.6% (HR 1.2 95% CI [0.49; 2.78] p = 0.74)]. However, the subgroup with a score of ≥ 3 presented a substantially higher occurrence of the composite endpoint [24.9% vs 37.6% (HR 1.7 95% CI [1.09; 2.53] p = 0.018)] and the single endpoints all-cause mortality [HR 1.7 95% CI [1.03; 2.88] p = 0.039)] and rehospitalization [HR 2.5 95% CI [1.14; 5.5] p = 0.022)], whereas the outcome of stroke did not differ (p = 0.13). Detailed results of the subgroups in the total cohort are presented in Table 3.
POAF in the LAA-amputation and control group
Among the control group, patients with POAF showed a worse outcome with a considerably higher frequency of the composite endpoint [17.3% vs 32.1% (HR 2.0 95% CI [1.15; 3.40] p = 0.013)] and the single endpoints all-cause mortality [8.0% vs 21.0% (HR 2.6 95% CI [1.26; 5.39] p = 0.010)] and rehospitalization [4.3% vs 11.1% (HR 2.8 95% CI [1.04; 7.69] p = 0.043)].
However, patients with LAA-amputation and POAF did not show a significantly higher rate of the composite and single endpoints [composite endpoint 23.2% vs 26.7% (HR 1.2 95% CI [0.68; 2.01] p = 0.57)]. Detailed results and survival curves are provided in Table 4 and Fig. 3.
Subgroup analysis in the LAA-amputation and control group
Regarding the subgroup analysis in the LAA-amputation and control group, no subgroup showed a noteworthy difference between patients with SR and POAF in the LAA-amputation cohort (Composite endpoint: age < 70 years p = 0.25; > 70 years p = 0.89; CHA2DS2-VASc-score < 3 p = 0.53; > 3 p = 0.62).
Additionally, in the control groups, regarding the subgroups with an assumed low risk by age (< 70 years) or CHA2DS2-VASc-Sore (< 3), no significant difference could be observed (composite endpoint: age < 70 years p = 0.58, CHA2DS2-VASc-score < 3 p = 0.78).
Contrary to that, a significant higher occurrence of the composite endpoint (18.4% vs. 35.2% HR 2.3 95% CI [1.18; 4.50] p = 0.015), driven by a higher rate of all-cause mortality (HR 3.1 95% CI [1.28; 7.32] p = 0.012) and rehospitalization (HR 5.4 95% CI [1.09; 26.97] p = 0.039) but no difference in the stroke rate (p = 0.57) could be detected in elderly patients (> 70 years) with POAF (Table 5).
In concordance to elderly patients, patients with a high CHA2DS2-VASc-score (> 3) and POAF presented a higher occurrence of the composite endpoint (19.2% vs. 40.0% HR 2.4 95% CI [1.27; 4.49] p = 0.007) with a higher percentage of all-cause mortality (HR 2.5 95% CI [1.10; 5.66] p = 0.028) and rehospitalization (HR 5.2 95% CI [1.38; 19.81] p = 0.015) but no difference in the stroke rate (p = 0.24).
Discussion
This is the first study to assess the long-term impact of LAA-amputation on the outcome in patients with new-onset POAF. Our analysis underlines the considerably worse outcome of patients developing POAF after OPCAB with regard to the composite endpoint of all-cause mortality, stroke, and rehospitalization. Furthermore, the outcome of patients with POAF who underwent LAA-amputation is not inferior compared to patients with maintained SR.
The rising evidence of stroke prevention by LAA-amputation in patients with atrial fibrillation (LAAOS III trial [13]) has led to an essential guideline recommendation for concomitant LAA-amputation [18]. Additionally, stroke prevention in patients with sinus rhythm undergoing cardiac surgery has also been demonstrated [14]. The results of this study go one step further and show that the prognosis of patients with POAF might be restored to that with maintained sinus rhythm. Furthermore, the safety of LAA-amputation with no extension of the cardiopulmonary bypass time and no increase of postoperative complications including bleeding, accompanied by a fast learning curve [19], strengthens the idea of expanding the indication for LAA-amputation in patients with risk factors assessed by the CHA2DS2-VASC-score. Even concerns about morphological effects on the left atrial geometry such as an increased intraatrial pressure causing dilation of the left atrium resulting in a higher rate of POAF [20], have been resolved [15].
Nevertheless, the conclusive result of this manuscript is the impaired outcome regarding all-cause mortality of patients who developed new-onset POAF after OPCAB surgery. This underlines the severity of an allegedly easy-to-handle postoperative complication and its influence on long-term patient prognosis. The impaired outcome of patients with POAF is in concordance with several studies demonstrating similarly severe mortality [21, 22]. Therefore, it is of utmost interest to identify risk factors for POAF and thus to develop strategies to avoid them.
In the current analysis, patients with POAF and no LAA-amputation showed an impaired outcome in relation to the composite endpoint, driven by all-cause mortality and rehospitalization. In contrast, POAF patients with LAA-amputation had no adverse effects compared to patients with maintained SR. On the other hand, there are hints that patients with maintained SR who received concomitant LAA-amputation have a slightly higher rate of all-cause mortality and rehospitalization without reaching statistical significance (Supplementary Table 2). A prolongation of hospitalization and an onset of heart failure symptoms have been described in literature [23]. In this regard, analysis of the often-discussed humoral impact of the LAA being the main source of atrial natriuretic peptide regulating renal clearance of electrolytes and water [24], is of utmost interest, and further research on this topic is warranted.
Additionally, in contrast to our results, Melduni et al. demonstrated no benefit regarding all-cause mortality and rehospitalization in patients undergoing concomitant LAA-amputation [20]. Hence, each case should be individually discussed, and the decision determined after careful risk factor evaluation for POAF.
Our analysis could proof the beneficial effect with regard to all-cause mortality and rehospitalization, particularly in patients with older age and an elevated stroke/comorbidity risk assessed by the CHA2DS2VASc-score, which itself includes older age. Thus, taking the current results into account, the CHA2DS2VASc-score, which is already being the main decision-making variable [6, 18], should be used for patients without preoperative AF, as well.
Patient selection has to always be a core point in prophylactic treatment approaches such as LAA-amputation, since the weight of responsibility is even greater, when recommending an additional procedure for treatment of a potential postoperative complication that could increase the risk of adverse outcomes, even though it might not manifest itself in the first place. When discussing a broad prophylactic recommendation, it is of utmost interest to establish the best possible technique for LAA amputation, which is still depending mostly on established habits, institutional experience, and economic considerations with the most commonly employed techniques of suturing, stapling and clipping [25]. This necessitates a levelheaded evidence-based discussion on general technical differences, patient-based differences and surgery-based differences (e.g. minimally invasive cardiac surgery, OPCAB, conventional cardiac surgery) to attain the required knowledge to form the surgeon’s preference, which is and will remain the decisive factor in making the choice.
Interestingly, in terms of the single endpoint stroke, no notable difference could be revealed. This raises the question of whether some stroke events are concealed within all-cause mortality without further investigation of the mortality cause. However, since several studies’ reporting’s range from “association” [3, 26, 27] to “no association”, the impact of POAF on long-term stroke risk needs to be further elucidated. Hsu et al. were able to demonstrate similar outcomes after POAF in a cohort of over 8000 patients with a higher mortality and rehospitalization rate but no increase in the stroke rate [28]. In contrast, sub-results of the EXCEL trial showed comparable results for patients with POAF with poor outcomes, indicating that POAF could be identified as an independent risk factor for stroke [29].
Limitations
Several limitations apply to our study. The main limitation of the analysis is the retrospective and single center study design. Furthermore, the anticoagulation strategy and patients’ compliance were not part of the follow-up, so a potential bias due to medical treatment differences cannot be ruled out. Nonetheless, similar to the LAAOS III cohorts [13] and following the guideline recommendations [6], all patients were treated with oral anticoagulation at least during hospitalization. Detailed information regarding LAA-amputation strategy was not given, however, the cohort assessed is an amputation-only cohort.
Conclusion
POAF is associated with a higher rate of the composite endpoint of all-cause mortality, stroke and rehospitalization. The combined endpoint in patients with LAA-amputation concomitant with OPCAB surgery developing new-onset POAF in a 5-year follow-up was not increased compared to a control cohort maintaining sinus rhythm.
Data availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
Abbreviations
- 95% CI:
-
95% Confidence interval
- AF:
-
Atrial fibrillation
- ASA:
-
American Society of Anesthesiologists
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CCS:
-
Canadian Cardiovascular Society
- CHA2DS2VASc:
-
Congestive heart failure, hypertension, age > 75 (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74 and sex category (female)
- CPR:
-
Cardiopulmonary resuscitation
- CRT:
-
Controlled randomized trial
- DVT:
-
Deep vein thrombosis
- ECLS:
-
Extracorporeal life support
- EF:
-
Ejection fraction
- HR:
-
Hazard ratio
- IABP:
-
Intra-aortic balloon pump
- IQR:
-
Interquartile range
- LAA:
-
Left atrial appendage
- NYHA:
-
New York Heart Association
- OPCAB:
-
Off-pump coronary artery bypass grafting
- OR:
-
Odds ratio
- PAPs:
-
Systolic pulmonary arterial pressure
- POAF:
-
Perioperative atrial fibrillation
- PS:
-
Propensity score
- SD:
-
Standard deviation
- SR:
-
Sinus rhythm
- STROBE:
-
STrengthening the Reporting of OBservational studies in Epidemiology
- TSH:
-
Thyroid stimulating hormone
- VT:
-
Ventricular tachycardia
References
Kaw R, Hernandez AV, Masood I, Gillinov AM, Saliba W, Blackstone EH (2011) Short- and long-term mortality associated with new-onset atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 141:1305–1312
Almassi GH, Wagner TH, Carr B, Hattler B, Collins JF, Quin JA et al (2015) Postoperative atrial fibrillation impacts on costs and one-year clinical outcomes: the Veterans Affairs Randomized On/Off Bypass Trial. Ann Thorac Surg 99:109–114
Thoren E, Wernroth ML, Christersson C, Grinnemo KH, Jideus L, Stahle E (2020) Compared with matched controls, patients with postoperative atrial fibrillation (POAF) have increased long-term AF after CABG, and POAF is further associated with increased ischemic stroke, heart failure and mortality even after adjustment for AF. Clin Res Cardiol 109:1232–1242
Echahidi N, Pibarot P, O’Hara G, Mathieu P (2008) Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol 51:793–801
Gillinov AM, Bagiella E, Moskowitz AJ, Raiten JM, Groh MA, Bowdish ME et al (2016) Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med 374:1911–1921
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al (2021) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 42:373–498
Chiang CE, Naditch-Brule L, Murin J, Goethals M, Inoue H, O’Neill J et al (2012) Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol 5:632–639
Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ et al (2014) Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129:837–847
Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P et al (2014) Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation 129:1568–1576
Blackshear JL, Odell JA (1996) Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 61:755–759
Damiano RJ Jr (2008) What is the best way to surgically eliminate the left atrial appendage? J Am Coll Cardiol 52:930–931
Ramlawi B, Abu Saleh WK, Edgerton J (2015) The left atrial appendage: target for stroke reduction in atrial fibrillation. Methodist Debakey Cardiovasc J 11:100–103
Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M et al (2021) Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 384:2081–2091
Gercek M, Borgermann J, Gercek M, Gummert J (2023) Left atrial appendage amputation concomitant to cardiac surgery in patients with sinus rhythm. Eur J Cardiothorac Surg. https://doi.org/10.1093/ejcts/ezad088
Gercek M, Ghabrial M, Glaubitz L, Kuss O, Aboud A, Paluszkiewicz L et al (2021) Impact of left atrial appendage amputation on left atrial morphology and rhythm after off-pump CABG. Thorac Cardiovasc Surg 71:273–281
Austin PC (2014) A comparison of 12 algorithms for matching on the propensity score. Stat Med 33:1057–1069
Kuss O (2013) The z-difference can be used to measure covariate balance in matched propensity score analyses. J Clin Epidemiol 66:1302–1307
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J et al (2022) 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 43:561–632
Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, Hohnloser SH et al (2005) Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 150:288–293
Melduni RM, Schaff HV, Lee HC, Gersh BJ, Noseworthy PA, Bailey KR et al (2017) Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality: a propensity score-matched analysis of 10,633 patients. Circulation 135:366–378
El-Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA et al (2010) New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol 55:1370–1376
Phan K, Ha HS, Phan S, Medi C, Thomas SP, Yan TD (2015) New-onset atrial fibrillation following coronary bypass surgery predicts long-term mortality: a systematic review and meta-analysis. Eur J Cardiothorac Surg 48:817–824
Mahmood E, Matyal R, Mahmood F, Xu X, Sharkey A, Chaudhary O et al (2020) Impact of left atrial appendage exclusion on short-term outcomes in isolated coronary artery bypass graft surgery. Circulation 142:20–28
Lakkireddy D, Turagam M, Afzal MR, Rajasingh J, Atkins D, Dawn B et al (2018) Left atrial appendage closure and systemic homeostasis: the LAA HOMEOSTASIS study. J Am Coll Cardiol 71:135–144
Salzberg SP, Emmert MY, Caliskan E (2017) Surgical techniques for left atrial appendage exclusion. Herzschrittmacherther Elektrophysiol 28:360–365
Ahlsson A, Fengsrud E, Bodin L, Englund A (2010) Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg 37:1353–1359
Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS et al (2014) Perioperative atrial fibrillation and the long-term risk of ischemic stroke. JAMA 312:616–622
Hsu JC, Huang CY, Chuang SL, Yu HY, Chen YS, Wang CH et al (2021) Long term outcome of postoperative atrial fibrillation after cardiac surgery—a propensity score-matched cohort analysis. Front Cardiovasc Med 8:650147
Kosmidou I, Chen S, Kappetein AP, Serruys PW, Gersh BJ, Puskas JD et al (2018) New-onset atrial fibrillation after PCI or CABG for left main disease: the EXCEL trial. J Am Coll Cardiol 71:739–748
Acknowledgements
We thank Miriam Switek for graphics.
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no competing interests.
Ethics committee approval
No: 2020-688_1; Date: 12.08.2022.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gerçek, M., Börgermann, J., Gummert, J. et al. Five-year-outcome of new-onset perioperative atrial fibrillation after left atrial appendage amputation concomitant with cardiac surgery. Clin Res Cardiol 112, 1800–1811 (2023). https://doi.org/10.1007/s00392-023-02255-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02255-8