Abstract
Background
The SYNTAX trial demonstrated negative impact of repeat revascularization (RR) on 5-year outcomes following PCI/CABG in patients with three-vessel(3VD) and/or left main coronary artery disease(LMCAD). We aimed to investigate the impact of RR within 5 years, on 10-year mortality in patients with 3VD and/or LMCAD after PCI/CABG.
Methods
The SYNTAXES study evaluated the vital status out to 10 years of patients with 3VD and/or LMCAD. Patients were stratified by RR within 5 years and randomized treatment. The association between RR within 5 years and 10-year mortality was assessed.
Results
A total of 330 out of 1800 patients (18.3%) underwent RR within 5 years. RR occurred more frequently after initial PCI than after initial CABG (25.9% vs. 13.7%, p < 0.001). Overall, 10-year mortality was comparable between patients undergoing RR and those not (28.2% vs. 26.1%, adjusted HR: 1.17, 95%CI 0.93–1.48, p = 0.187). In the PCI arm, RR was associated with a trend toward higher 10-year mortality (adjusted HR: 1.29, 95%CI 0.97–1.72, p = 0.075), while in the CABG arm, the trend was opposite (adjusted HR: 0.74, 95%CI 0.46–1.20, p = 0.219). Among patients requiring RR, those who underwent PCI as initial revascularization had a higher risk of 10-year mortality compared to initial CABG (33.5% vs. 17.6%, adjusted HR: 2.09, 95%CI 1.21–3.61, p = 0.008).
Conclusion
In the SYNTAXES study, RR within 5 years had no impact on 10-year all-cause death in the population overall. Among patients requiring any repeat procedures, 10-year mortality was higher after initial treatment with PCI than after CABG. These exploratory findings should be investigated with larger populations in future studies.
Trial registration
URL: https://www.clinicaltrials.gov; SYNTAXES Unique identifier: NCT03417050. URL: https://www.clinicaltrials.gov; SYNTAX Unique identifier: NCT00114972.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The higher rate of additional revascularization required after percutaneous coronary intervention (PCI) compared to coronary artery bypass graft (CABG) has been one of the Achilles' heels of PCI, and this was first addressed by the introduction and routine use of bare metal stents [1,2,3], with further improvements seen in contemporary practice by their replacement with ever improving drug-eluting stents (DES) [4]. Nevertheless, recent trials comparing PCI to CABG continue to underscore the superiority of CABG in the reduction of this adverse event [4,5,6,7].
Repeat revascularization (RR) remains a potential complication in both PCI and CABG patients. Although RR is considered an adverse outcome or failure of the initial treatment, as a strategy it often offers an efficient treatment associated with a reduction in morbidity or mortality [8]. Some authors argue that RR cannot be considered a reliable outcome indicator because of a large number of confounding factors that contribute to the event, ranging from variable indications, the differences in the availability of revascularization targets, to patient preferences [9]. However, the clinical relevance of RR is not negligible, and its negative impact on major adverse events and quality of life has been consistently reported. The SYNTAX trial reported higher rates of the composite endpoint of death, stroke, and myocardial infarction (MI) at 5 years, among patients who were initially randomized to PCI and then underwent secondary revascularization compared to those who did not. A similar, but less impactful, trend was reported among those randomized to CABG [10]. More recently, analyses related to RR in patients with left main (LM) lesions from the EXCEL trial have reaffirmed the association between RR and the risk of 3-year all-cause and cardiovascular mortality after both PCI and CABG [11].
Despite these data, there is currently no evidence as to whether these trends are maintained or amplified long term. Specifically, the impact of RR on all-cause death beyond 5 years has not been fully elucidated. The present study aimed to investigate the impact of RR within 5 years of PCI or CABG on all-cause death among patients with 3VD and/or LMCAD beyond the original 5-year follow-up of SYNTAX trial.
Methods
Study population
The SYNTAX study design and the 5-year results have been published previously [5, 12, 13]. The SYNTAX trial completed patient follow-up up to 5 years [13]. The SYNTAXES study was an investigator-driven initiative that extended follow-up using vital status up to 10 years [14]. The extended follow-up was funded by German Heart Research Foundation (GHF; Frankfurt am Main, Germany) and performed in accordance with local regulations of each participating center and complied with the Declaration of Helsinki.
Endpoints and definitions
The primary endpoint of SYNTAXES study was all-cause death at 10 years. RR was collected within the first 5 years, but no longer recorded after 5 years in SYNTAXES study. The present study is a secondary analysis from the SYNTAXES study assessing the association between RR procedures within 5 years and 10-year all-cause death. In case of patients undergoing more than one RR (regardless of the type), the time-to-first-event was considered for statistical analysis.
The association between the type of RR (PCI or CABG) and 10-year all-cause death was also explored. Patients undergoing RR were categorized into three subgroups according to the type of additional intervention: RR-PCI (patients underwent ≥ 1 RR only by means of PCI), RR-CABG (patients underwent ≥ 1 RR only by means of CABG), and RR-PCI/CABG (patients underwent ≥ 2 RR by means of both PCI and CABG).
Initial PCI and initial CABG refer to the primary revascularization procedure driven by randomization. A staged revascularization procedure was permitted in the SYNTAX trial protocol, provided it was performed ≤ 72 h of the index procedure and during the same hospital stay [12], and these were not considered RR.
Statistical analysis
Continuous variables are expressed as mean and SD and compared using Student t test or Wilcoxon rank sum test as appropriate. Categorical data are reported as counts and percentages, and compared using Chi-square or Fisher’s exact test, when appropriate. Event rates were generated using Kaplan–Meier estimates in time-to-first-event analyses and were compared using log-rank test. Proportional hazard assumption was checked and was not violated in the present study. Therefore, Cox proportional hazard model was applied, and hazard ratio (HR) with 95% confidence interval (CI) was computed. The association of RR with the risk for 10-year all-cause death was evaluated using multivariable Cox models. The covariables in the adjusted Cox models included age, sex, body mass index, prior MI, chronic obstructive pulmonary disease, peripheral vascular disease, medically treated diabetes, chronic kidney disease, congestive heart failure, and anatomic SYNTAX score. All these variables were selected based on prior knowledge of the association of those variables with clinical outcomes [15]. A two-sided p value < 0.05 was considered to be statistically significant. All analyses were performed using SPSS Statistics, version 25 (IBM Corp., Armonk, 281 N.Y., USA).
Results
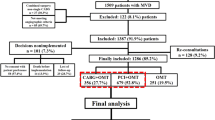
A total of 330 out of 1800 patients (18.3%) underwent ≥ 1 RR within 5 years of their initial procedures, which amounted to 454 RR procedures (390 additional PCI and 64 additional CABG). The 330 patients undergoing RR were made up of 237, 69, 17, and 7 patients having 1, 2, 3, and 4 RR procedures, respectively (Fig. 1). According to the type of RR, patients were categorized as follows: 266 patients in RR-PCI group, 45 in RR-CABG group, and 19 in RR-PCI/CABG group (Fig. 1).
Baseline characteristics
Baseline characteristics in patients with vs. without RRs are reported in Tables S1 and S2. In PCI arm, patients undergoing RRs were more likely to be diabetic and had a higher rate of incomplete revascularization; while in CABG group, they had a lower rate of prior MI and a lower logistic EuroSCORE compared to those who did not have RRs (Table S2).
Impact of RR within 5 years on long-term outcomes
In overall population, 10-year all-cause death was comparable between patients who underwent a RR within 5 years and those who did not (28.2% vs. 26.1%, HR: 1.10, 95%CI 0.87–1.38, log-rank p = 0.430, Fig. 2A). In PCI arm, patients who underwent any RR had a numerically higher rate of 10-year all-cause death compared to those who did not (33.5% vs. 26.8%, HR: 1.30, 95%CI 0.99–1.72, log-rank p = 0.056, Fig. 2B). By contrast, in CABG population, patients who had any RR had a numerically lower rate of 10-year mortality compared to those who did not (17.6% vs. 25.5%, HR: 0.66, 95%CI 0.41–1.06, log-rank p = 0.085, Fig. 2C). Of note, the findings were unchanged following adjustment for baseline confounders (Tables 1 and Table 2).
Impact of initial revascularization (PCI or CABG) on long-term outcomes in patients who had RR within 5 years
The crude rate of 10-year mortality among patients who underwent RR in the first 5 years was higher when the primary mode of revascularization was PCI compared to CABG (33.5% vs. 17.6%, HR: 2.08, 95%CI 1.26–3.45, log-rank p = 0.004, Fig. 3), with similar findings seen after adjustment for confounding factors (adjusted HR: 2.09, 95%CI 1.21–3.61, p = 0.008, Fig. 4).
Risk of 10-year all-cause death in PCI and CABG arms, among patients who had RR within 5 years and those who did not, stratified by subgroups
In terms of long-term survival, there was a trend for a treatment interaction between the initial modality of revascularization and having a RR (p-interaction = 0.059, Fig. 4). In subgroup analyses among patients who had a RR within 5 years, significant treatment-by-subgroup interactions were observed only in the 3VD/LMCAD subgroups in terms of 10-year mortality (Fig. 4).
Impact of type of RR within 5 years on 10-year mortality
Independent of initial treatment modality, the risk of 10-year all-cause death was the highest when additional revascularization was performed with CABG only (RR-CABG, n = 45), followed by PCI only (RR-PCI, n = 266), and then both PCI and CABG (RR-PCI/CABG, n = 19) (45.8% vs. 26.1% vs. 15.8%, respectively, log-rank p = 0.008).
Multivariable analysis
After adjustment for confounding factors, while RR was not an independent risk factor for 10-year all-cause death, RR with CABG was (adjusted HR: 1.87, 95%CI 1.19–2.95, p = 0.007, Table 1), whereas RR with PCI was not (Table 1). The high risk of RR with CABG for 10-year all-cause death was mainly contributed by patients whose primary revascularization was with CABG (adjusted HR: 6.94, 95%CI 2.79–17.30, p < 0.001, Table 2). The association between the type of revascularization and 10-year all-cause death in initial PCI arm and initial CABG arm is shown in Table 2.
SYNTAX score II 2020 for predicting 10-year all-cause death in patients with RR
Figure 5 shows ranked individual differences (n = 330) in predicted mortality for patients having RR following primary revascularization with PCI (blue dashed line) or CABG (red dashed line). Notably, 229 patients had a predicted mortality which was higher after PCI than CABG; following this, a cross-over point in predicted mortalities (equipoise) was reached, and beyond this, the predicted mortality in the remaining 101 patients was lower following PCI than CABG. The solid line in Fig. 5 depicts, in a spline regression (LOESS) [16], the observed mortality after PCI or CABG. Notably, the solid lines depicting the observed mortalities following either PCI or CABG cross-over the 295th ranked patient suggesting equipoise in vital prognosis after either PCI or CABG for that specific patient.
The individual difference between predicted mortality (dashed lines) using the SYNTAX Score II 2020 and the individual observed mortality (solid lines), between initial PCI and initial CABG in patients with repeat revascularization. Blue dashed line represents the predicted mortality after PCI; Red dashed line represents the predicted mortality after CABG; Blue solid line represents the observed mortality after PCI; Red solid line represents the observed mortality after CABG
Discussion
The present study investigated the association between RR within 5 years of the index PCI or CABG and 10-year all-cause death, among patients with 3VD and/or LMCAD in SYNTAX trial. The main findings are:
-
(1)
RR within 5 years did not have impact on 10-year all-cause death.
-
(2)
Patients requiring RR had a significantly higher rate of 10-year all-cause death when their primary revascularization was with PCI compared to CABG.
-
(3)
Overall, RR was not an independent predictor of 10-year all-cause death. However, RR with CABG was, unlike RR with PCI. The high risk of RR with CABG for 10-year all-cause death was mainly contributed by patients whose primary revascularization was with CABG (RR was a redo CABG).
-
(4)
The SYNTAX score II 2020 was able to identify those patients who would benefit the most from either CABG or PCI.
Numerous studies have demonstrated that RR has a negative impact on short- and mid-term outcomes. In patients who underwent elective primary revascularization with isolated CABG, RR with PCI had a poorer prognosis at a median follow-up of 58 months, compared with those who did not [17]. A pooled patient-level analysis from 21 randomized PCI trials demonstrated that target lesion revascularization (TLR) after PCI increased mortality at a median follow-up of 37 months, which was mainly driven by higher rates of MI occurring after TLR [18]. In another patient-level pooled analysis of 1001 patients, TLR was associated with a higher rate of 5-year mortality compared with those without TLR [19]. Similarly in EXCEL trial, the need for a RR increased the risk of 3-year all-cause death after both PCI and CABG (p-interaction = 0.85) [11]. These studies only had medium-term follow-up of 3 to 5 years, the impact of RR on very long-term all-cause death was previously unknown.
In line with the 5-year results of SYNTAX trial [10], we observed a comparable rate of 10-year all-cause death between patients with and without a RR. Whether the impact of RR is related to the mode of primary revascularization is uncertain. In EXCEL trial, among patients having a RR, 3-year all-cause death was numerically higher if the index procedure was with PCI rather than CABG (10.4% vs. 9.1%) [11]. In SYNTAX trial, at 5 years, among patients who underwent RR, those initially randomized to PCI as opposed to CABG had significantly higher rates of the composite of death, MI, or subsequent RR (57.4% vs. 38.4%, p = 0.003), and a trend for higher mortality (20.2% vs. 13.9%, p = 0.095) [10]; with the difference in mortality significant by 10 years (Fig. 3). As the value of SYNTAX scores in predicting clinical outcomes [20], we explored SYNTAX score II 2020 for predicting 10-year mortality in patients with RR. Similarly, we demonstrated that the majority of patients (n = 295) having a RR have a higher rate of 10-year mortality after primary revascularization with PCI than CABG (Fig. 5). SYNTAX score II 2020 clearly identifies those individuals who derive a treatment-specific survival benefit.
There are many possible reasons for these findings. In SYNTAX study, RRs after PCI were mainly target vessel revascularizations, and to a lesser extent treatment of de novo lesions. Disease progression and restenosis appear to be more aggressive after index revascularization with PCI than CABG [10], which may be due to the detrimental effect that coronary stenting has on endothelial function in the distal coronary bed [21, 22]. In addition, bystander enemies, such as diabetes, may contribute to endothelial dysfunction and aggressive disease progression, in particular, in patients who underwent initial PCI, likely influencing their long-term prognosis. As previously reported, indeed, in PCI group, diabetic patients were significantly more frequent among those requiring additional revascularization than those not (34.1% vs. 22.8%, p < 0.001) [10]. In contrast, bypass grafts to the mid-part of coronary vessels not only removes the vulnerability of proximal lesions, but also potentially offers biological protection and prophylaxis against the development of de novo disease [23].
In addition, initial treatment with CABG offers complete revascularization more frequently than PCI, which may result in a greater protective effect on long-term prognosis [24]. Consistently, PCI patients undergoing RR had a significantly higher incidence of incomplete revascularization than those not requiring RR, whereas such a difference was not observed in the index CABG group (Table S2). This suggests that incomplete revascularization during CABG may not have significant impact on RR risk, probably because most vessels that were not revascularized are either chronically occluded or too small, making their treatment irrelevant. However, the vein graft failure over a 10-year period is high, and might attenuate these benefits. Indeed, multi-arterial CABG is associated with lower mortality [25], which may also partially contribute to the higher rate of 10-year all-cause death after primary revascularization with PCI compared to CABG.
The impact of type of RR on mortality remains uncertain, with previous results inconsistent. A patient-level pooled analysis demonstrated that the type of RR did not affect mortality after TLR of LMCAD [19]. In EXCEL trial, RR using CABG was strongly associated with increased 3-year mortality [11]; in our analysis, after adjustment for confounders, RR-CABG was the only independent predictor of 10-year all-cause death. Of note, the high risk of RR with CABG for 10-year all-cause death was mainly contributed by patients whose primary revascularization was with CABG (Table 2). Furthermore, in our study, the risk of mortality was higher following secondary revascularization with CABG, compared to PCI or both PCI and CABG. Similarly, Locker et al. showed that redo CABG increased 30-day mortality compared to RR with PCI in patients with previous CABG [26]. Given that this result was observed after adjustment, our findings indicate that RR with CABG is a high-risk procedure especially for those with prior CABG, and should, therefore, be considered carefully with the patients. However, considering there were very limited patients who underwent RR with CABG in isolation or following PCI, our findings should be considered as exploratory and hypothesis generating. In addition, RR-CABG patients usually present severe 3VD either not amenable with PCI or where PCI already failed with a large area of myocardium at risk, or yet present non-functioning LIMA-LAD bypass that is a predictor of long-term survival, characteristics that generate biases and preclude definite conclusions. Nevertheless, in patients with previous CABG, the ESC Guideline recommends PCI as the first choice for RR if technically feasible, rather than redo CABG [27]. The recently published ACC/AHA Guideline also recommends that in patients with previous CABG with a patent LIMA to the LAD who need RR, if PCI is feasible, it is reasonable to choose PCI over CABG [28].
At 5 years in SYNTAX trial, patients who underwent RR by PCI had a numerically higher rate of all-cause death compared to those who did not, when their initial revascularization was also by PCI (16.6% vs. 13.2%, p = 0.26), whereas the opposite was seen after initial CABG (6.3% vs. 12.1%, p = 0.084) [10]. At 10 years, results were similar, with a trend for increased mortality following primary and secondary revascularization with PCI, and a significantly lower risk when primary CABG was followed by PCI (Table 2). In contrast in EXCEL trial, RR with PCI or CABG were both associated with an increased risk for all-cause death regardless of the initial revascularization approach (PCI or CABG), with no significant interaction observed between the initial revascularization procedure and any type of RR [11]. Ultimately, these inconsistent findings need to be explored in adequately powered clinical studies.
Limitations
The present study is a post hoc analysis of SYNTAXES trial and may not have adequate statistical power. The main limitation is that RR was no longer recorded after 5 years, we cannot be confident that our findings are reflective of RR in its entirety up to 10 years. Second, the number of patients who underwent RR was limited, especially for those who had repeat CABG or both procedures. Our analysis may lead to the likelihood of spurious findings, all results should be considered exploratory and hypothesis generating. Third, angiographic follow-up was not routinely performed in SYNTAX trial and may underestimate the true rate of RR, especially in patients with silent ischemia. Moreover, not all confounders may have been identified in our multivariable Cox model which assessed the association between RR and all-cause death. Furthermore, the endpoint in SYNTAXES study was all-cause death only; however, death has been considered the most robust and unbiased index for clinical assessment, and is less likely to be affected by ascertainment bias [29]. Finally, the stent-type and peri-interventional therapy used for repeat revascularization were not available. SYNTAX trial enrolled patients with 3VD and/or LMCAD and patients received PCI with first-generation DES; hence, our results should not be extrapolated to general CAD patients in contemporary practice. Further investigations, including latest generation stents and guideline-oriented medical therapy, are warranted.
Conclusion
In patients with 3VD and/or LMCAD undergoing PCI or CABG, RR within 5 years was not associated with the risk of 10-year all-cause death in the whole population. Among patients requiring any repeat procedures, a higher death rate was observed after primary revascularization with PCI than CABG. The SYNTAX score II 2020 can identify individuals who derive a treatment-specific survival benefit. RR with CABG was associated with an increased risk of 10-year all-cause death especially in patients with previous CABG. These exploratory findings should be investigated in larger populations of patients either pooled retrospectively or enrolled in prospective future studies.
Data availability
Data will be made available upon request in adherence to transparency conventions in medical research and through reasonable requests to the corresponding author.
References
Serruys PW, Ong AT, van Herwerden LA, Sousa JE, Jatene A, Bonnier JJ et al (2005) Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 46(4):575–581
Rodriguez A, Bernardi V, Navia J, Baldi J, Grinfeld L, Martinez J et al (2001) Argentine randomized study: coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple-vessel disease (ERACI II): 30-day and one-year follow-up results. ERACI II Investigators. J Am Coll Cardiol 37(1):51–58
Hueb W, Soares PR, Gersh BJ, César LA, Luz PL, Puig LB et al (2004) The medicine, angioplasty, or surgery study (MASS-II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one-year results. J Am Coll Cardiol 43(10):1743–1751
Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ (2004) A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet 364(9434):583–591
Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ et al (2009) Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 360(10):961–972
Thiele H, Neumann-Schniedewind P, Jacobs S, Boudriot E, Walther T, Mohr FW et al (2009) Randomized comparison of minimally invasive direct coronary artery bypass surgery versus sirolimus-eluting stenting in isolated proximal left anterior descending coronary artery stenosis. J Am Coll Cardiol 53(25):2324–2331
Park SJ, Kim YH, Park DW, Yun SC, Ahn JM, Song HG et al (2011) Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med 364(18):1718–1727
Taniwaki M, Stefanini GG, Silber S, Richardt G, Vranckx P, Serruys PW et al (2014) 4-Year clinical outcomes and predictors of repeat revascularization in patients treated with new-generation drug-eluting stents: a report from the RESOLUTE All-Comers trial (A Randomized Comparison of a Zotarolimus-Eluting Stent With an Everolimus-Eluting Stent for Percutaneous Coronary Intervention). J Am Coll Cardiol 63(16):1617–1625
Lamelas P, Belardi J, Whitlock R, Stone GW (2019) Limitations of repeat revascularization as an outcome measure: JACC review topic of the week. J Am Coll Cardiol 74(25):3164–3173
Parasca CA, Head SJ, Milojevic M, Mack MJ, Serruys PW, Morice MC et al (2016) Incidence, characteristics, predictors, and outcomes of repeat revascularization after percutaneous coronary intervention and coronary artery bypass grafting: the SYNTAX Trial at 5 years. JACC Cardiovasc Interv 9(24):2493–2507
Giustino G, Serruys PW, Sabik JF, Mehran R, Maehara A, Puskas JD et al (2020) Mortality after repeat revascularization following PCI or CABG for left main disease: the EXCEL trial. JACC Cardiovasc Interv 13(3):375–387
Ong AT, Serruys PW, Mohr FW, Morice MC, Kappetein AP, Holmes DR Jr et al (2006) The SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study: design, rationale, and run-in phase. Am Heart J 151(6):1194–1204
Mohr FW, Morice MC, Kappetein AP, Feldman TE, Ståhle E, Colombo A et al (2013) Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet (London, England) 381(9867):629–638
Thuijs D, Kappetein AP, Serruys PW, Mohr FW, Morice MC, Mack MJ et al (2019) Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 394:1325–1334
Pocock SJ, McMurray JJV, Collier TJ (2015) Statistical controversies in reporting of clinical trials: part 2 of a 4-part series on statistics for clinical trials. J Am Coll Cardiol 66(23):2648–2662
Wang R, Serruys PW, Gao C, Hara H, Takahashi K, Ono M et al (2021) Ten-year all-cause death after percutaneous or surgical revascularization in diabetic patients with complex coronary artery disease. Eur Heart J. https://doi.org/10.1016/j.jacc.2021.09.959
Genichi S, Takeshi S, Tatsuhiko K (2011) Impact of repeated percutaneous coronary intervention on long-term survival after subsequent coronary artery bypass surgery. J Cardiothorac Surg 6:107
Palmerini T, Della Riva D, Biondi-Zoccai G, Leon MB, Serruys PW, Smits PC et al (2018) Mortality following nonemergent, uncomplicated target lesion revascularization after percutaneous coronary intervention: an individual patient data pooled analysis of 21 randomized trials and 32,524 patients. JACC Cardiovasc Interv 11(9):892–902
Wiebe J, Kuna C, Ibrahim T, Losl M, Cassese S, Kufner S et al (2020) Long-term prognostic impact of restenosis of the unprotected left main coronary artery requiring repeat revascularization. JACC Cardiovasc Interv 13(19):2266–2274
Askin L, Tanriverdi O (2022) The clinical value of syntax scores in predicting coronary artery disease outcomes. Cardiovasc Innov Appl 6(4):197–208
Kim JW, Suh SY, Choi CU, Na JO, Kim EJ, Rha SW et al (2008) Six-month comparison of coronary endothelial dysfunction associated with sirolimus-eluting stent versus Paclitaxel-eluting stent. JACC Cardiovasc Interv 1(1):65–71
Hamilos M, Sarma J, Ostojic M, Cuisset T, Sarno G, Melikian N et al (2008) Interference of drug-eluting stents with endothelium-dependent coronary vasomotion: evidence for device-specific responses. Circ Cardiovasc Interv 1(3):193–200
Taggart DP (2013) CABG or stents in coronary artery disease: end of the debate? Lancet (London, England) 381(9867):605–607
Takahashi K, Serruys PW, Gao C, Ono M, Wang R, Thuijs D et al (2021) Ten-year all-cause death according to completeness of revascularization in patients with three-vessel disease or left main coronary artery disease: insights from the SYNTAX extended survival study. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.046289
Chikwe J, Sun E, Hannan EL, Itagaki S, Lee T, Adams DH et al (2019) Outcomes of second arterial conduits in patients undergoing multivessel coronary artery bypass graft surgery. J Am Coll Cardiol 74(18):2238–2248
Locker C, Greiten LE, Bell MR, Frye RL, Lerman A, Daly RC et al (2019) Repeat coronary bypass surgery or percutaneous coronary intervention after previous surgical revascularization. Mayo Clin Proc 94(9):1743–1752
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U et al (2019) 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 40(2):87–165
Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM et al (2021) ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021:CIR0000000000001038
Lauer MSBE, Young JB, Topol EJ (1999) Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol 34:618–620
Funding
Open Access funding provided by the IReL Consortium. The SYNTAX Extended Survival study was supported by the German Foundation of Heart Research (Frankfurt am Main, Germany). The SYNTAX trial, during 0–5-year follow-up, was funded by Boston Scientific Corporation (Marlborough, MA, USA). Both sponsors had no role in the study design, data collection, data analyses, and interpretation of the study data, nor were involved in the decision to publish the final manuscript. The principal investigators and authors had complete scientific freedom. Science Foundation Ireland (Research Professorship Grant 15/RP/2765) grant to Dr William Wijns supports Drs R. Wang, M. Lunardi and C. Gao.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Burzotta reports speaker’s fees from Abiomed, Abbott, Terumo and Medtronic. Dr. Hara reports a grant for studying overseas from Japanese Circulation Society, a grant-in-Aid for JSPS Fellows, a grant-in-aid from Japan Foundation for Applied Enzymology, and a grant from Fukuda Foundation for Medical Technology. Dr. Davierwala reports speaker’s fees from Medtronic. Dr. Davierwala holds the Angelo & Lorenza De Gasperis Chair in Cardiovascular Surgery Research, an endowed joint Hospital-University Chair among University Health Network (UHN), the University of Toronto (UofT), and UHN Foundation. Dr. Head reports working as a full-time employee of Medtronic outside the scope of this work. Dr. Kappetein reports working as an employee of Medtronic, outside the submitted work. Dr. Serruys reports personal fees from Biosensors, Micel Technologies, Sinomedical Sciences Technology, Philips/Volcano, Xeltis, and HeartFlow, outside the submitted work. Dr. Wijns reports working as Co-founder of Argonauts, an innovation facilitator. All other authors have no disclosures.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, R., Lunardi, M., Hara, H. et al. Impact of repeat revascularization within 5 years on 10-year mortality after percutaneous or surgical revascularization. Clin Res Cardiol 112, 1302–1311 (2023). https://doi.org/10.1007/s00392-023-02211-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-023-02211-6