Abstract
Objectives
To evaluate potential risk factors for stroke or transient ischemic attacks (TIA) and to test the feasibility and efficacy of a Fabry-specific stroke risk score in Fabry disease (FD) patients without atrial fibrillation (AF).
Background
FD patients often experience cerebrovascular events (stroke/TIA) at young age.
Methods
159 genetically confirmed FD patients without AF (aged 40 ± 14 years, 42.1% male) were included, and risk factors for stroke/TIA events were determined. All patients were followed up over a median period of 60 (quartiles 35–90) months. The pre-defined primary outcomes included new-onset or recurrent stroke/TIA and all-cause death.
Results
Prior stroke/TIA (HR 19.97, P < .001), angiokeratoma (HR 4.06, P = .010), elevated creatinine (HR 3.74, P = .011), significant left ventricular hypertrophy (HR 4.07, P = .017), and reduced global systolic strain (GLS, HR 5.19, P = .002) remained as independent risk predictors of new-onset or recurrent stroke/TIA in FD patients without AF. A Fabry-specific score was established based on above defined risk factors, proving somehow superior to the CHA2DS2-VASc score in predicting new-onset or recurrent stroke/TIA in this cohort (AUC 0.87 vs. 0.75, P = .199).
Conclusions
Prior stroke/TIA, angiokeratoma, renal dysfunction, left ventricular hypertrophy, and global systolic dysfunction are independent risk factors for new-onset or recurrent stroke/TIA in FD patients without AF. It is feasible to predict new or recurrent cerebral events with the Fabry-specific score based on the above defined risk factors. Future studies are warranted to test if FD patients with high risk for new-onset or recurrent stroke/TIA, as defined by the Fabry-specific score (≥ 2 points), might benefit from antithrombotic therapy. Clinical trial registration HEAL-FABRY (evaluation of HEArt invoLvement in patients with FABRY disease, NCT03362164).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fabry disease (FD) is an X-linked inherited disorder characterized by deficiency of lysosomal α-galactosidase A, which affects many body organs including the skin, eyes, gastrointestinal system, kidney, heart, peripheral and central nervous system due to progressive accumulation of globotriaosylceramide (Gb3) and other glycosphingolipids within lysosome carrying cells [1,2,3]. Patients with FD often experience cerebrovascular events such as stroke or transient ischemic attacks (TIA) at young age, even before FD diagnosis and in the absence of other clinical events. Reported incidence of stroke ranged from 7 to 48% in FD patients [4, 5]. The “silent progression” nature of stroke in a relevant portion of FD patients thus requires timely recognition, risk stratification and efficient treatment. A better understanding of the risk factors for stroke in patients with FD might lead to improved prevention and therapeutic strategies not only in FD patients, but potentially also in other multisystemic diseases, storage disorders, and/or (secondary) cardiomyopathies. Currently, the risk factors of stroke/TIA in FD patients without AF remain largely unknown.
Numerous scoring methods have been established for prediction of stroke in patients with atrial fibrillation (AF) [6], including the CHADS2, CHA2DS2-VASc, or the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) risk score [7]. The CHA2DS2-VASc score is currently the most commonly used score for estimating the risk of stroke in patients with non-rheumatic AF [8]. Clinical utility of this risk score was also suggested in heart failure patients with reduced ejection fraction without AF [9, 10]. It remains unknown now if the CHA2DS2-VASc score can also be meaningfully used to predict stroke risk in patients with inherited diseases with myocardial damage and cardio-cerebral vascular complications, such as FD.
Congestive heart failure patients face an increased risk of stroke [11]. It is thus reasonable to speculate that conventional and modern left ventricular (LV) functional markers derived from echocardiography, i.e., ejection fraction (EF) and global systolic strain (GLS) might be useful to predict stroke risk in FD patients. In the present study, we tested the hypothesis that a Fabry-specific score based on pre-defined risk factors for stroke/TIA might be feasible to evaluate the risk of stroke in FD patients without AF. The purposes of this study were (i) to determine potential risk factors for stroke/TIA in FD patients without AF; (ii) to evaluate whether the CHA2DS2-VASc score can predict cerebrovascular events in FD patients without AF; (iii) to develop and test the feasibility and efficacy of a modified Fabry-specific risk score method based on defined risk factors in a large FD patient cohort.
Methods
Study population
This retrospective investigation on stroke/TIA risk of FD patients included patients from a prospective single-center observational study (HEAL-FABRY, ClinicalTrials.gov Identifier: NCT03362164). In brief, patients with genetically confirmed FD referred to the Fabry Center for Interdisciplinary Treatment Würzburg (FAZIT) between 2001 and June 2015 were eligible for this study; all patients with follow-up for > 2 years were screened. Sixteen patients with AF were excluded and 159 patients were included for the final analysis (Suppl. Fig. 1). All patients underwent extensive on-site characterization of disease severity, related organ involvement and concomitant diseases. Demographic data, medical history and laboratory data were collected from the annual visit at FAZIT including review of all related external medical records. Conventional echocardiographic measurements and speckle tracking imaging (STI) analysis were performed off-line by two experienced investigators. The study was conducted in accordance to the Declaration of Helsinki, and approved by the local Ethics Committee at the University of Würzburg. Written informed consent was obtained from all patients.
Stoke and TIA diagnosis
Prior history of stroke and TIA were identified from medical records at baseline visit. New-onset or recurrent stroke was defined according to the AHA/ASA definition of stroke in 2013, based on pathological, imaging or other objective evidence of infarction. In case of the absence of this evidence, the persistence of symptoms of at least 24 h or until death remained a method to define stroke [12]. TIA was defined as a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischemia, with typical clinical symptoms and without evidence of acute infarction, based on a comprehensive evaluation including clinical, laboratory, and imaging modalities [13].
CHA2DS2-VASc score
The CHA2DS2-VASc score was calculated based on the original description by Lip et al. [8].
Electrocardiogram
Standard resting 12-channel ECG was performed in all patients. A total of 134 (84.3%) patients received a 24-h Holter ECG monitoring at baseline visit. An atrial high-rate episode was defined as an atrial tachyarrhythmia with an atrial rate ≥ 180 beats/min lasting ≥ 5 min.
Conventional and speckle tracking echocardiography
A standard echocardiographic examination was performed on a Vivid 7/9e echo machine (GE Vingmed, Horten, Norway). Cardiac structural and functional measurements were performed off-line after initial image acquisition according to recommended guidelines [14]. STI analysis was performed using EchoPAC software (GE, Horten, Norway) [15]. Global longitudinal peak systolic strain (GLS) was calculated by averaging strain values of all LV 18 segments.
Cardiac magnetic resonance imaging
Cardiac magnetic resonance (CMR) imaging was performed using a 1.5 T full body magnetic resonance scanner (Magnetom Symphony Quantum/Avanto, Siemens Medical Systems, Erlangen, Germany) as previously described [16]. Late gadolinium enhancement (LGE) images were acquired 15 min after intravenous injection of 0.2 mmol/kg gadopentetate dimeglumine, using T1-weighted inversion recovery imaging sequences (field of view 240 × 320 mm2, matrix size 165 × 256, slice thickness 8 mm, echo time 3.4 ms, repetition time 7.5 ms). A stack of multiple short-axis views covering the entire left ventricle were applied to detect changes in the LV myocardial tissue. All LV segments were evaluated for the presence of myocardial fibrosis by two radiologists who were blinded to all clinical data. Areas with signal intensity above the average of the normal myocardium plus 2 standard deviations were defined as LGE positive. For analysis of right ventricular (RV) function, short-axis views covering the RV were analyzed with a commercially available post-processing software for CMR (CVI 42®, Circle Cardiovascular Imaging Inc., Calgary, Canada). For the quantitative assessment of the RVEF, the RV volume was determined in systole and diastole.
Diagnosis of Fabry cardiomyopathy
Diagnostic criteria by echocardiography or/and CMR on Fabry cardiomyopathy were as follows: (1) LV concentric hypertrophy with a LV wall thickness ≥ 12 mm or asymmetric left ventricular hypertrophy (LVH) characterized by significant thickening at the septum and thinning at the posterior wall, detected by echocardiography and CMR; (2) reduced LVEF (< 55%); (3) myocardial replacement fibrosis detected by CMR LGE imaging. Genetically proven FD patients who matched any of these three criteria were defined as Fabry cardiomyopathy [17].
Statistical analysis
Continuous variables are expressed as mean ± standard deviation or median (quartiles). Categorical variables are presented as count and percentage. Differences on continuous data between groups were compared using unpaired Student’s t test or Mann–Whitney U test, as appropriate. Categorical data were compared using a similar approach employing Chi-square or Fisher’s exact test. Given cumulative hazard analyses, Kaplan–Meier curves were plotted and compared using the log rank test. All variables with P < .05 in Tables 1 and 2 were tested as potential risk factors of new-onset or recurrent stroke/TIA in the univariable Cox proportional hazard regression models as well as in the multivariable models adjusted for age and sex, presented as the hazard ratio (HR) with 95% confidence intervals (CI). The cut-off values of creatinine, left ventricular posterior wall thickness (LVPWd) and GLS for predicting new-onset or recurrent stroke/TIA events were derived from receiver operating characteristic curves analysis by maximizing the sum of the sensitivity and specificity. The significance of the difference between the areas under two receiver operating characteristic curves was compared using Hanley and McNeil’s method. Statistical significance was defined as P < .05 (two-tailed test). Statistical analysis was performed using IBM SPSS, version 23 for Windows (IBM Corp., New York, USA).
Results
Mean age of the total population was 40 ± 14 (range 10–75) years and 42.1% (70/159) of patients were male. Within the period from birth to the last follow-up visit, 38 subjects (23.9%) experienced stroke and/or TIA events (19 ischemic strokes and 35 TIAs). Stroke/TIA occurred in 19 men (27.1%, 12 ischemic strokes and 16 TIAs), and 19 women (21.3%, 7 ischemic strokes and 19 TIAs). From all individuals with cerebral events, 34 subjects had a history of stroke/TIA before baseline echocardiographic examination, 12 had recurrent stroke/TIA and 4 experienced new-onset stroke/TIA, 11 patients died (including 7 cardiac deaths) during a median of 60 (quartiles 35–90) months follow-up. All strokes were ischemic and 2 patients suffered from stroke with residual deficits.
As shown in Table 1, patients were significantly older in the events group compared to the no-events group (45 ± 13 vs. 39 ± 15 years, P = .020). Mean age at first onset of stroke events was 40 ± 13 (range 14–68) years. Creatinine level and the prevalence of angiokeratoma (52.6 vs. 32.2%) were significantly higher in the events group than in the no-events group (both P < .05). Two patients (1.7%) in the no-events group received antiplatelet medication due to coronary artery disease, and 7 patients (18.4%) in the events group received antiplatelet medication (6 with aspirin only and 1 with additional clopidogrel).
The prevalence of ST–T alterations was significantly higher in the events group than in the no-events groups (56.8 vs. 37.3%, P = .036). Other ECG parameters were comparable between groups. On 24-h Holter ECG monitoring, an atrial high-rate episode was documented in 2 out of 134 (1.5%) patients who did not suffer from stroke/TIA, while none was tested positive for silent AF episodes.
A total of 136 patients underwent a CMR examination (Table 1). The remaining patients (n = 23) did not because of the presence of contraindications to LGE imaging such as end-stage renal dysfunction, implanted cardiac pacemakers or defibrillators, or claustrophobia, etc. In 51 out of 56 (91.1%) LGE positive patients LGE positive areas were identified in the typical location at the basal inferolateral and/or inferior wall [events group n = 15 (100%) vs. no-events group n = 36 (87.8%), P = .309]. Right ventricular function could be measured in 133 patients by CMR with a mean RVEF of 55 ± 10%. No difference in RVEF was found comparing the no-events and events groups (54 ± 10 vs. 56 ± 11%, P = .312).
Fabry cardiomyopathy was identified in 80 (50.3%) patients and the proportion of cardiomyopathy was significantly higher in the events group compared to the no-events group (65.8 vs. 45.5%, P = .029). Definitive cardiac-related genotype mutations (N215S) were detected in 12 (7.5%) patients. The percentage of left ventricular hypertrophy (LVH) tended to be higher in these patients compared to patients without the N215S mutation [41.7% (5/12) vs. 27.2% (40/147), P = .322].
Echocardiography data (Table 2) showed that LV septal, LVPWd, and LV mass index were significantly higher, and GLS was remarkably lower in the events group than in the no-events group (all P < .05). LVEF was similar between groups (66 ± 7 vs. 65 ± 8%).
Cox regression analysis showed that history of prior stroke/TIA was the only independent predictor (adjusted HR 19.97, 95% CI 5.61–71.08, P < .001) while age, female sex, history of congestive heart failure, hypertension, diabetes, and vascular disease were not predictors of stroke/TIA in this Fabry cohort. Further analysis showed that elevated creatinine level (≥ 1.0 mg/dl, HR 3.74, 95% CI 1.35–10.35, P = .011), significant LV hypertrophy with LVPWd > 14 mm (HR 4.08, 95% CI 1.29–12.87, P = .017), reduced GLS (< 13.5%, HR 5.19, 95% CI 1.83–14.71, P = .002), and the presence of angiokeratoma (HR 4.06, 95% CI 1.41–11.69, P = .010) remained as independent risk predictors of stroke/TIA in FD patients (Suppl. Table 1 and Suppl. Figure 2). It is to note, although the prevalence of coronary artery disease and NYHA class were significantly higher in FD patients with stroke/TIA, Cox regression analysis showed that coronary artery disease (HR 1.063, P = .953) and NYHA class (HR 1.145, P = .858) were not independent predictors of new-onset or recurrent stroke/TIA in this patient cohort.
Based on above findings, we established a Fabry-specific score for risk stratification of stroke in FD patients (Table 3): prior stroke/TIA (2 points), angiokeratoma (1 point), creatinine ≥ 1.0 mg/dl (1 point), LVPWd > 14 mm (1 point) and GLS < 13.5% (1 point).
Supplementary Table 2 and Supplementary Fig. 3 show the incidence rates of new-onset or recurrent stroke/TIA, all-cause death, and combined events during follow-up assessed by the CHA2DS2-VASc score and Fabry-specific score in FD patients with individual risk score value. The risk of developing new-onset stroke or recurrent, all-cause mortality and combined events increased in proportion to increasing scores.
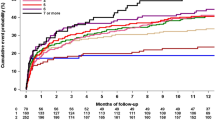
There was an obvious trend that the predictive power for new-onset or recurrent stroke/TIA of the Fabry-specific score is superior to the CHA2DS2-VASc score, although this trend did not reach statistical significance in this FD cohort (AUC 0.87, 95% CI 0.79–0.94 vs. AUC 0.75, 95% CI 0.62–0.87, P = .199; Fig. 1a). The predicting efficacy for the all-cause mortality was comparable by both score methods (Fig. 1b).
As shown in Fig. 2, patients with high stroke/TIA risk (≥ 2 points) defined by both CHA2DS2-VASc score and Fabry-specific score, were associated with significantly increased incidence rate of new or recurrent stroke/TIA as compared to patients with low or intermediate risk. The annualized event rate of stroke/TIA was 0.78, 0.35 and 4.13 per 100 patient-years as assessed by the CHA2DS2-VASc score scheme for patients with low, intermediate and high risk, and was 0, 0.90 and 6.41 per 100 patient-years by Fabry-specific score scheme for patients with low, intermediate, and high risk (Suppl. Table 3).
Discussion
The main findings of this study are, (i) the CHA2DS2-VASc score is feasible for predicting new-onset or recurrent stroke/TIA in FD patients without AF; (ii) prior stroke/TIA, angiokeratoma, creatinine ≥ 1.0 mg/dl, LVPWd > 14 mm, and GLS < 13.5% are independent risk factors for new-onset or recurrent stroke/TIA in FD patients without AF; (iii) the predicting efficacy of Fabry-specific score based on the defined risk factors in these FD patients is superior to classic CHA2DS2-VASc score on risk stratification of new-onset or recurrent stroke/TIA in FD patients without AF.
Cerebrovascular events in FD
Cerebrovascular complication is the major cause of early morbidity and mortality in patients with FD. Ischemic stroke and TIA are the most prevalent cerebrovascular events in FD patients. Previous studies reported a wide range (from 7 to 48%) of the incidence of stroke in FD patients [18]. A large cohort study with 2446 patients in the Fabry Registry US demonstrated that the stroke incidence in FD was 6.9% in men and 4.3% in women, and increased with age [4], which was markedly higher than that in the general US population across all age categories and ∼20% of FD patients experience a first stroke below the age of 30 years [4]. The prevalence of stroke/TIA was 23.9% in our cohort and 23.7% patients experienced the first stroke with the age of < 30 years. All cerebrovascular events in our cohort were of ischemic origin, which is basically in line with previous findings in that 87% of the first stroke event was of ischemic origin [4]. The underlying pathogenic mechanisms of stroke in Fabry disease remain elusive now, possibly linked with an interaction between the progressive neuronal accumulation of glycosphingolipids and specific cellular proteins, leading to cellular and organ dysfunction [19]. Lysosomal accumulations of GL-3 could induce oxidative stress and promote the formation of reactive oxygen species, thus causing sustained dilation of the cerebral vessels, which might result in cerebral vasculature endothelial dysfunction and increase the vulnerability of cerebral vasculature [19, 20]. Cerebral blood vessel abnormalities in FD are characterized by cerebral glucose metabolism impairment due to endothelial proliferation or vessel stenosis. Increased platelet reactivity and altered cerebrovascular reactivity because of autonomic dysfunction might also attribute to vasculopathy of stroke in FD [21].
Risk assessment of stroke/TIA in FD patients using the CHA2DS2-VASc risk score
The CHA2DS2-VASc score is feasible to predict stroke risk in heart failure patients without AF. Our study results demonstrated for the first time that this score is also useful for predicting new-onset stroke risk in FD patients without AF (AUC 0.749, 95% CI 0.624–0.874, P = .001). The most powerful contributing element among all co-risk factors, however, was the presence of prior stroke history, while additional risk factors including age and gender only played a minor role in this FD entity.
Risk assessment of stroke/TIA in FD patients by the Fabry-specific score
Previous stroke/TIA was included in this Fabry-specific score, since this is a major contributing factor for risk assessment of new-onset or recurrent stroke.
Another major player in the Fabry-specific score is the presence of angiokeratoma of FD patients. Angiokeratoma is a common and early dermatological manifestation in FD. In the early years, term of “Angiokeratoma corporis diffusum” was used to define Fabry disease [22, 23]. It may be the earliest physical sign of FD, and often occurs in children between the ages of 5 and 15 years of Fabry patients [24]. Angiokeratoma is a kind of benign skin lesion characterized by proliferation of dilated blood vessels in the upper dermis, caused by accumulation of Gb3 in dermal endothelial cells, followed by vascular ectasia [25, 26]. Information regarding the prevalence of angiokeratoma in FD remains largely limited. Larralde et al. reported a quite high angiokeratoma incidence of ∼80% in FD [24]. A retrospective clinical and CMR study with a small cohort (n = 43) also revealed a high incidence of angiokeratoma (88%) in male FD patients [27]. In contrast, angiokeratoma was detected in only one (1%) adult female FD patient (< 40 years) in a recent clinical study with a cohort of 191 adult and pediatric Fabry patients carrying the IVS4 + 919G > A mutation [28]. Data from 261 adult female FD patients from 6 German Fabry centers showed that 39% of female FD patients had angiokeratoma. In female FD patients, angiokeratoma is more frequently documented in patients with nonsense mutations as compared to patients with missense mutations (61 vs. 40%, P < .05) [29]. In our cohort, angiokeratoma is documented in one-third of FD patients (total 37%, 19% in female vs. 60% in male, P < .001), and the prevalence of angiokeratoma is significantly higher in patients with stroke/TIA than in those without stroke/TIA (52.6 vs. 32.2%, P = .023), especially in male patients with stroke/TIA than in those without stroke/TIA (90 vs. 49%, P = .002). Results from our group and others thus indicate that prevalence of angiokeratoma in FD patients varies significantly depending on mutations in the GLA gene and gender and this skin lesion occurs more commonly in men with FD than in women with FD. A previous study from our group found that this gender difference in prevalence of angiokeratoma may be related to the higher distal skin Gb3 load in male FD patients as compared to female FD patients and to health controls based on skin biopsy results [30]. Due to the close association between the presence of angiokeratoma and stroke/TIA events in our FD cohort, and the finding that angiokeratoma served as an independent factor of new-onset or recurrent stroke/TIA based on the results of the multivariable Cox regression models, angiokeratoma is included in the Fabry-specific score. To our knowledge, this is the first report on the association between angiokeratoma and stroke risk in Fabry disease. Although this skin lesion is not non-specific for Fabry disease, pathologically, the underlying mechanisms are both related to accumulation of Gb3 in dermal vasculature or cerebral vasculature, thus, it is tempting to speculate that visible skin angiokeratoma might be linked with stroke/TIA due to the common pathological changes of the dermal and cerebral vasculature.
STI derived global systolic strain (GLS) has been extensively applied to assess and monitor LV systolic global function as well as to predict outcome in various cardiovascular disorders in recent years [31,32,33]. Previous studies have demonstrated that GLS could provide additional information on cerebrovascular risk burden [34, 35]. In line with above finding, results from the multivariable Cox regression models in our FD cohort also indicated that reduced (< 13.5%) GLS value is capable of predicting the risk of new-onset or recurrent stroke/TIA and is associated with about fivefold increased stroke/TIA risk than GLS ≥ 13.5% in our FD cohort. It is thus reasonable to include this fundamental parameter reflecting LV systolic function in our Fabry-specific score.
LVH has been recognized as a risk factor for stroke [36,37,38,39]. Likewise, a CMR study revealed that the LV size was related to stroke in a multiethnic cohort, suggesting that concentric ventricular remodeling predicted incident stroke [40]. Left ventricular concentric hypertrophy is a common imaging finding in Fabry disease and is reported in up to 50% of males and one-third of females. In our cohort, significant LVH (LVPWd > 14 mm) is detected in 26% of patients with stroke/TIA (37% of males vs. 16% of females, P = .269), whereas detected in about 4% of patients without stroke/TIA (7% of males vs. 4% of females, P = .459), suggesting that significant LVH serves as a potential risk factor for new-onset or recurrent stroke/TIA in patients with FD.
Renal involvement is a typical disease characteristic of FD patients. The link between chronic kidney disease and cerebrovascular disease has become more apparent [41]. Our results also showed that the creatinine level was significantly higher in the events group than in the no-events group and creatinine ≥ 1.0 mg/dl serves as an independent predictor of new-onset or recurrent stroke/TIA in FD patients without AF.
Predicting efficacy of the risk of new-onset or recurrent stroke/TIA by the Fabry-specific score
In the present study, we explored the potential role of easy-to-obtain clinical [Fabry associated skin markers (angiokeratoma), renal dysfunction (elevated creatinine), and prior stroke/TIA] and echocardiographic parameters (LVPWd and GLS) in new-onset or recurrent stroke/TIA risk assessment of FD patients. The new score presents more precise predictive performance for new-onset or recurrent stroke/TIA risk assessment in FD. Diagnostic power of high risk ≥ 2 points according to the Fabry-specific score for predicting new-onset or recurrent stroke/TIA was: specificity 71%, sensitivity 88%; while the specificity was 57%, sensitivity was 88% according to the CHA2DS2-VASc risk score. In addition, 38% of Fabry patients were identified as low stroke risk, 28% as intermediate risk, and 34% as high risk (≥ 2 points) according to the Fabry-specific score. In contrast, these values assessed by CHA2DS2-VASc score were 16, 36 and 48%, respectively. Thus, the Fabry-specific score fairly well represents the real-world risk scenario in this FD cohort for new-onset or recurrent stroke/TIA, while the CHA2DS2-VASc score underestimated the FD patients with low stroke risk and overestimated the FD patients with high stroke risk. AUC values of Fabry-specific score (AUC 0.87) is also superior to the classic CHA2DS2-VASc score (AUC 0.75).
In the present study, prior events of TIA were included in the Fabry-specific score as a criterion with two points and compared with the CHA2DS2-VASc score for the predictive efficacy on risk of stroke/TIA in this patient cohort. The predicting efficacy was better using the Fabry-specific score than the CHA2DS2-VASc score in this study setting; however, the CHA2DS2-VASc score was originally developed to predict stroke events and not TIA. This difference in the two scores needs to be kept in mind when interpreting the current data analysis.
Clinical implication
Despite manifold reasons for cerebro- or cardiovascular events have been revealed until today, predictability and optimum treatment remain controversial [42,43,44,45,46], stressing the need for further research in this regard. Our results demonstrate that the Fabry-specific score is useful for the risk assessment of new-onset or recurrent stroke/TIA in FD patients without AF. Since all new-onset or recurrent stroke/TIA events in this cohort were of ischemic origin, and the specificity and sensitivity for predicting new-onset or recurrent stroke/TIA by the high risk (Fabry score ≥ 2 points) were around 70–80%. It is therefore reasonable to monitor FD patients based on this score, since those with high calculated risk might benefit from additional therapy such as antithrombotic medication in an effort to reduce future events of new-onset or recurrent stroke/TIA in FD patients.
Study limitations
The Fabry-specific stroke risk assessment is established on the risk factors defined in a single-center cohort with a relatively small sample size. The predicting efficacy of the new Fabry-specific score should be tested and validated in other FD cohort. Furthermore, future multicenter registry studies are needed to define the efficacy and safety of antiplatelet or anticoagulant regimens in FD patients with and without AF.
Conclusion
Prior stroke/TIA, angiokeratoma, LVPWd > 14 mm, creatinine ≥ 1.0 mg/dl and GLS < 13.5% are independent risk factors for new-onset or recurrent stroke/TIA in FD patients without AF. The new predicting score based on the above defined risk factors in this FD patient cohort is superior to classic CHA2DS2-VASc score on predicting new-onset or recurrent stroke/TIA in FD patients without AF. FD patients with high risk for new-onset or recurrent ischemic stroke/TIA might therefore benefit from the regular and intensive antithrombotic therapy.
References
Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR (2003) Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med 138:338–346
Mehta A, Ginsberg L (2005) Natural history of the cerebrovascular complications of Fabry disease. Acta Paediatr Suppl 94:24–27 (discussion 29 - 10)
Oder D, Uceyler N, Liu D, Hu K, Petritsch B, Sommer C, Ertl G, Wanner C, Nordbeck P (2016) Organ manifestations and long-term outcome of Fabry disease in patients with the GLA haplotype D313Y. BMJ Open 6:e010422
Sims K, Politei J, Banikazemi M, Lee P (2009) Stroke in Fabry disease frequently occurs before diagnosis and in the absence of other clinical events: natural history data from the Fabry Registry. Stroke 40:788–794
Mehta A, Ricci R, Widmer U, Dehout F, Garcia de Lorenzo A, Kampmann C, Linhart A, Sunder-Plassmann G, Ries M, Beck M (2004) Fabry disease defined: baseline clinical manifestations of 366 patients in the Fabry Outcome Survey. Eur J Clin Invest 34:236–242
Brainin M, Heiss W-D (2014) Textbook of stroke medicine. Cambridge University Press, Cambridge
Oldgren J, Hijazi Z, Lindback J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Granger CB, Hylek EM, Lopes RD, Siegbahn A, Yusuf S, Wallentin L (2016) Performance and validation of a novel biomarker-based stroke risk score for atrial fibrillation. Circulation 134:1697–1707
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137:263–272
Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY (2015) Assessment of the CHA2DS2-VASc Score in predicting ischemic stroke, thromboembolism, and death in patients with heart failure with and without atrial fibrillation. JAMA 314:1030–1038
Ye S, Qian M, Zhao B, Buchsbaum R, Sacco RL, Levin B, Di Tullio MR, Mann DL, Pullicino PM, Freudenberger RS, Teerlink JR, Mohr JP, Graham S, Labovitz AJ, Estol CJ, Lok DJ, Ponikowski P, Anker SD, Lip GY, Thompson JL, Homma S (2016) CHA2 DS2 -VASc score and adverse outcomes in patients with heart failure with reduced ejection fraction and sinus rhythm. Eur J Heart Fail 18:1261–1266
Kang SH, Kim J, Park JJ, Oh IY, Yoon CH, Kim HJ, Kim K, Choi DJ (2017) Risk of stroke in congestive heart failure with and without atrial fibrillation. Int J Cardiol 248:182–187
Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H (2013) Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44:870–947
Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL (2009) Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 40:2276–2293
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270
Hu K, Liu D, Nordbeck P, Cikes M, Stork S, Kramer B, Gaudron PD, Schneider A, Knop S, Ertl G, Bijnens B, Weidemann F, Herrmann S (2015) Impact of monitoring longitudinal systolic strain changes during serial echocardiography on outcome in patients with AL amyloidosis. Int J Cardiovasc Imaging 31:1401–1412
Kramer J, Niemann M, Liu D, Hu K, Machann W, Beer M, Wanner C, Ertl G, Weidemann F (2013) Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur Heart J 34:1587–1596
Seydelmann N, Liu D, Kramer J, Drechsler C, Hu K, Nordbeck P, Schneider A, Stork S, Bijnens B, Ertl G, Wanner C, Weidemann F (2016) High-sensitivity troponin: a clinical blood biomarker for staging cardiomyopathy in fabry disease. J Am Heart Assoc 5:pii:e002839
Kolodny E, Fellgiebel A, Hilz MJ, Sims K, Caruso P, Phan TG, Politei J, Manara R, Burlina A (2015) Cerebrovascular involvement in Fabry disease: current status of knowledge. Stroke 46:302–313
Moore DF, Kaneski CR, Askari H, Schiffmann R (2007) The cerebral vasculopathy of Fabry disease. J Neurol Sci 257:258–263
Shen JS, Meng XL, Moore DF, Quirk JM, Shayman JA, Schiffmann R, Kaneski CR (2008) Globotriaosylceramide induces oxidative stress and up-regulates cell adhesion molecule expression in Fabry disease endothelial cells. Mol Genet Metab 95:163–168
Rombach SM, Twickler TB, Aerts JM, Linthorst GE, Wijburg FA, Hollak CE (2010) Vasculopathy in patients with Fabry disease: current controversies and research directions. Mol Genet Metab 99:99–108
Pompen AW, Ruiter M, Wyers HJ (1947) Angiokeratoma corporis diffusum (universale) Fabry, as a sign of an unknown internal disease; two autopsy reports. Acta Med Scand 128:234–255
Karen JK, Hale EK, Ma L (2005) Angiokeratoma corporis diffusum (Fabry disease). Dermatol Online J 11:8
Larralde M, Boggio P, Amartino H, Chamoles N (2004) Fabry disease: a study of 6 hemizygous men and 5 heterozygous women with emphasis on dermatologic manifestations. Arch Dermatol 140:1440–1446
Lidove O, Jaussaud R, Aractingi S (2006) Dermatological and soft-tissue manifestations of Fabry disease: characteristics and response to enzyme replacement therapy. In: Mehta A, Beck M, Sunder-Plassmann G (eds) Fabry disease: perspectives from 5 years of FOS. PharmaGenesis, Oxford
Yokota M, Koji M, Yotsumoto S (1995) Histopathologic and ultrastructural studies of angiokeratoma corporis diffusum in Kanzaki disease. J Dermatol 22:10–18
Buechner S, Moretti M, Burlina AP, Cei G, Manara R, Ricci R, Mignani R, Parini R, Di Vito R, Giordano GP, Simonelli P, Siciliano G, Borsini W (2008) Central nervous system involvement in Anderson-Fabry disease: a clinical and MRI retrospective study. J Neurol Neurosurg Psychiatry 79:1249–1254
Auray-Blais C, Lavoie P, Boutin M, Ntwari A, Hsu TR, Huang CK, Niu DM (2017) Biomarkers associated with clinical manifestations in Fabry disease patients with a late-onset cardiac variant mutation. Clin Chim Acta 466:185–193
Lenders M, Hennermann JB, Kurschat C, Rolfs A, Canaan-Kuhl S, Sommer C, Uceyler N, Kampmann C, Karabul N, Giese AK, Duning T, Stypmann J, Kramer J, Weidemann F, Brand SM, Wanner C, Brand E (2016) Multicenter Female Fabry Study (MFFS)—clinical survey on current treatment of females with Fabry disease. Orphanet J Rare Dis 11:88
Uceyler N, Schroter N, Kafke W, Kramer D, Wanner C, Weidemann F, Sommer C (2016) Skin Globotriaosylceramide 3 load is increased in men with advanced fabry disease. PLoS ONE 11:e0166484
Weidemann F, Strotmann JM (2008) Use of tissue Doppler imaging to identify and manage systemic diseases. Clin Res Cardiol 97:65–73
Prinz C, Farr M, Hering D, Horstkotte D, Faber L (2010) Reduction in ECG abnormalities and improvement of regional left ventricular function in a patient with Fabry’s disease during enzyme-replacement therapy. Clin Res Cardiol 99:53–55
Huttin O, Marie PY, Benichou M, Bozec E, Lemoine S, Mandry D, Juilliere Y, Sadoul N, Micard E, Duarte K, Beaumont M, Rossignol P, Girerd N, Selton-Suty C (2016) Temporal deformation pattern in acute and late phases of ST-elevation myocardial infarction: incremental value of longitudinal post-systolic strain to assess myocardial viability. Clin Res Cardiol 105:815–826
Olsen FJ, Pedersen S, Jensen JS, Biering-Sorensen T (2016) Global longitudinal strain predicts incident atrial fibrillation and stroke occurrence after acute myocardial infarction. Medicine (Baltimore) 95:e5338
Munk K, Andersen NH, Terkelsen CJ, Bibby BM, Johnsen SP, Botker HE, Nielsen TT, Poulsen SH (2012) Global left ventricular longitudinal systolic strain for early risk assessment in patients with acute myocardial infarction treated with primary percutaneous intervention. J Am Soc Echocardiogr 25:644–651
Verdecchia P, Porcellati C, Reboldi G, Gattobigio R, Borgioni C, Pearson TA, Ambrosio G (2001) Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation 104:2039–2044
Selvetella G, Notte A, Maffei A, Calistri V, Scamardella V, Frati G, Trimarco B, Colonnese C, Lembo G (2003) Left ventricular hypertrophy is associated with asymptomatic cerebral damage in hypertensive patients. Stroke 34:1766–1770
O’Neal WT, Almahmoud MF, Qureshi WT, Soliman EZ (2015) Electrocardiographic and echocardiographic left ventricular hypertrophy in the prediction of stroke in the elderly. J Stroke Cerebrovasc Dis 24:1991–1997
Wang S, Xue H, Zou Y, Sun K, Fu C, Wang H, Hui R (2014) Left ventricular hypertrophy, abnormal ventricular geometry and relative wall thickness are associated with increased risk of stroke in hypertensive patients among the Han Chinese. Hypertens Res 37:870–874
Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR (2008) The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 52:2148–2155
Hojs Fabjan T, Hojs R (2014) Stroke and renal dysfunction. Eur J Intern Med 25:18–24
Andrikopoulos G, Tzeis S, Terentes-Printzios D, Varounis C, Vlachopoulos C, Mantas I, Patsilinakos S, Lampropoulos S, Olympios C, Kartalis A, Manolis A, Gotsis A, Triposkiadis F, Tsaknakis T, Goudevenos I, Kaprinis I, Pras A, Vasiliou F, Skoumpourdis E, Sakka G, Draganigos A, Vardas P (2016) Impact of income status on prognosis of acute coronary syndrome patients during Greek financial crisis. Clin Res Cardiol 105:518–526
Zeus T, Ketterer U, Leuf D, Dannenberg L, Bönner F, Wagstaff R, Gliem M, Jander S, Kelm M, Polzin A (2016) Safety of percutaneous coronary intervention in patients with acute ischemic stroke/transient ischemic attack and acute coronary syndrome. Clin Res Cardiol 105:356–363
Fox KM, Tai MH, Kostev K, Hatz M, Qian Y, Laufs U (2017) Treatment patterns and low-density lipoprotein cholesterol (LDL-C) goal attainment among patients receiving high- or moderate-intensity statins. Clin Res Cardiol 107:380–388 (Epub ahead of print)
Yadlapati A, Groh C, Malaisrie SC, Gajjar M, Kruse J, Meyers S, Passman R (2016) Efficacy and safety of novel oral anticoagulants in patients with bioprosthetic valves. Clin Res Cardiol 105:268–272
Seeger J, Bothner C, Dahme T, Gonska B, Scharnbeck D, Markovic S, Rottbauer W, Wöhrle J (2016) Efficacy and safety of percutaneous left atrial appendage closure to prevent thromboembolic events in atrial fibrillation patients with high stroke and bleeding risk. Clin Res Cardiol 105:225–229
Funding
This study was supported by grants from the Bundesministerium für Bildung und Forschung (BMBF 01EO1504 MO.2 and MO.6) and Genzyme Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
P. Nordbeck, C. Wanner and F. Weidemann received speaker and/or advisory board honoraria from Amicus, Genzyme and Shire corporations. Research grants were given to the Institution by Genzyme and Shire corporations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, D., Hu, K., Schmidt, M. et al. Value of the CHA2DS2-VASc score and Fabry-specific score for predicting new-onset or recurrent stroke/TIA in Fabry disease patients without atrial fibrillation. Clin Res Cardiol 107, 1111–1121 (2018). https://doi.org/10.1007/s00392-018-1285-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1285-4