Abstract
Background
Locally advanced rectal cancer (LARC) typically involves neoadjuvant chemoradiotherapy (nCRT) followed by surgery (total mesorectal excision, TME). While achieving a complete pathological response (pCR) is a strong indicator of a positive prognosis, the specific benefits of adjuvant chemotherapy after pCR remain unclear. To address this knowledge gap, we conducted a systematic review and meta-analysis to assess the potential advantages of adjuvant therapy in patients who achieve pCR.
Methods
In this study, we searched Medline, Embase, and Web of Science databases for relevant research. We focused on binary outcomes, analyzing them using odds ratios (ORs) with 95% confidence intervals (CIs). To account for potential variability between studies, all endpoints were analyzed with DerSimonian and Laird random-effects models. We assessed heterogeneity using the I2 statistic and employed the R statistical software (version 4.2.3) for all analyses.
Results
Thirty-four studies, comprising 31,558 patients, were included. The outcomes demonstrated a significant difference favoring the AC group in terms of overall survival (OS) (HR 0.75; 95% CI 0.60–0.94; p = 0.015; I2 = 0%), and OS in 5 years (OR 1.65; 95% CI 1.21–2.24; p = 0.001; I2 = 39%). There was no significant difference between the groups for disease-free survival (DFS) (HR 0.94; 95% CI 0.76–1.17; p = 0.61; I2 = 17%), DFS in 5 years (OR 1.19; 95% CI 0.82–1.74; p = 0.36; I2 = 43%), recurrence-free survival (RFS) (HR 1.10; 95% CI 0.87–1.40; p = 0.39; I2 = 0%), and relapse-free survival (OR 1.08; 95% CI 0.78–1.51; p = 0.62; I2 = 0%).
Conclusion
This systematic review and meta-analysis found a significant difference in favor of the ACT group in terms of survival after pCR. Therefore, the administration of this treatment as adjuvant therapy should be encouraged in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is a leading cause of cancer morbidity and mortality worldwide [1]. Notably, younger patients with CRC often present with aggressive tumor types diagnosed at advanced stages [2, 3]. Among CRC cases, locally advanced rectal cancer (LARC) represents a significant challenge in clinical practice [4]. Currently, the standard treatment for LARC consists of neoadjuvant chemoradiotherapy (NCRT) followed by total mesorectal excision (TME) [5, 6]. Microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) tumors, identified by specific tests (PCR sequencing or immunohistochemistry), are present in a small subset of LARC patients (1–3%) [7, 8]. In these patients, neoadjuvant immunotherapy has shown remarkable complete clinical response (cCR) rates [7, 8]. However, the majority of LARC cases involve microsatellite stable (MSS) or mismatch repair-proficient (pMMR) tumors. For these patients, a multimodal approach combining (chemo)radioimmunotherapy holds greater promise than immunotherapy alone. While NCRT offers advantages such as improved local tumor control and sphincter preservation, its effectiveness is variable, with only a minority of patients (10–30%) achieving complete pathological response (pCR) [6, 9, 10]. This translates to a high rate of tumor recurrence (25–40%) [11].
Complete pathological response (pCR), defined as the absence of viable tumor cells in the surgical specimen after neoadjuvant chemoradiotherapy (NCRT), is a significant milestone in the treatment of locally advanced rectal cancer (LARC)[12]. This absence of tumor cells is a strong indicator of favorable prognosis, associated with a considerable improvement in survival and a reduction in the risk of disease recurrence [13, 14]. pCR is associated with an approximately 70% reduction in the risk of local tumor recurrence and a 50% decrease in the risk of death from colorectal cancer (CRC) [15,16,17].
Although pCR has a positive prognostic value, the need for adjuvant chemotherapy (ACT) after NCRT for patients with pCR is still not fully understood. Current treatment guidelines, such as those from the National Comprehensive Cancer Network (NCCN) [18], recommend ACT for all patients who have received NCRT, regardless of their response; however, the impact of ACT on long-term survival outcomes in patients with pCR is inconclusive, and previous studies have shown conflicting results. Thus, this systematic review and meta-analysis aims to comprehensively evaluate the impact of ACT on the treatment of LARC patients who achieve pCR after NCRT.
Methods
Protocol and registration
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [19] (PRISMA Checklist, Supplementary Tables S1 and S2). The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO), National Institute for Health and Care Research (NIHR) on December 13th, 2023, with registration number CRD42024519913.
This meta-analysis investigated the impact of adjuvant therapy (ACT) on treatment outcomes in patients with rectal cancer. To select the studies, we used the PICO question: Population (P), patients diagnosed with rectal cancer; Intervention (I), receipt of adjuvant therapy (ACT); Comparison (C), patients who did not receive it (no-ACT); Outcomes (O), evaluation of the impact of ACT on overall survival (OS), disease-free survival (DFS), and relapse-free survival (RFS).
Eligibility criteria
Studies that met the following eligibility criteria were included: (1) patients diagnosed with rectal cancer who received neoadjuvant chemoradiotherapy; (2) studies comparing ACT with No-ACT after pCR; (3) outcome of interest: OS, DFS, and RFS. We excluded studies with overlapping populations, single-arm clinical trials, and studies without results of interest.
We therefore sought to answer the following question: Doess(ACT) improve the outcomes of patients with rectal cancer that achieved pCR after neoadjuvant chemotherapy and subsequent surgery?
Search strategy
On January 25, 2024, a systematic search was conducted across three databases: PubMed, Embase, and Web of Science. The search strategy employed MeSH terms, the details of which are provided in Table S4 of the Supplementary Material. In an effort to include additional studies, the references of the articles included, as well as systematic reviews of the literature, were evaluated. An alert was set up in each database to notify of any newly published studies that matched the search criteria. The studies identified in the databases and in the references of the articles were integrated into the reference management software (EndNote®, version X7, Thomson Reuters, Philadelphia, USA). Duplicate articles were excluded both automatically and manually. The titles and abstracts of the articles found in the databases were independently analyzed by two reviewers (F.C.A.d.M. and R.M.R.B).
Data extraction and endpoints
We extracted the following information: (1) study design; (2) age; (3) gender; (3) adjuvant chemotherapy; (4) adjuvant radiotherapy; (5) country of study; (6) overall survival (OS); (7) disease-free survival (DFS); and relapse-free survival (RFS).
We define the following: overall survival (OS), time elapsed from diagnosis to death from any cause [20]; disease-free survival (DFS), time elapsed from diagnosis to first local or regional recurrence, distant metastasis, or death from any cause [21]; and relapse-free survival (RFS), time elapsed from diagnosis to first local or regional recurrence [22].
Two authors (F.A.K. and F.C.A.M.) collected baseline characteristics and pre-specified outcome data. Whenever available, we consulted the full protocol of each study to check the objectives, population, and other relevant information about the design and conduct of the study. For publications reporting results from the same study, the most recent or complete publication was considered.
Risk of bias assessment
The quality assessment of observational studies was performed using the Newcastle–Ottawa Scale (NOS), in which studies are scored on a 0 to 9 scale according to selection, comparability, and exposure criteria [23]. Two authors (F.C.A.M. and R.M.R.B.) independently conducted the risk of bias assessment, and disagreements were resolved by consensus. Funnel-plot analyses were employed to examine publication bias [24].
We used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach to assess the overall quality of the evidence obtained by the included RCTs [25]. This framework categorizes evidence quality into four levels based on the assessment of the methodological limitations, inconsistency, imprecision, indirectness, and publication bias: very low, low, moderate, and high. For this evaluation, we used GRADEpro GDT software (Copyright © 2020, McMaster University and Evidence Prime Inc., USA).
Statistical analysis
Risk ratio (OR) was used to analyze the binary outcomes, with 95% confidence intervals (CI). We consider OR > 1 favoring the control (No-ACT) group and OR < 1 favoring the intervention group (ACT). The Cochrane Q-test and I2 statistics were used to assess heterogeneity; p values > 0.10 and I2 values > 25% were considered to indicate significance for heterogeneity [26]. The Sidik–Jonkman estimator was used to calculate the tau2 variance between studies [27]. We used DerSimonian and Laird random-effect models for all endpoints [28]. Publication bias was explored using Egger’s linear regression test [29]. Statistical analyses were performed using R statistical software, version 4.2.3 (R Foundation for Statistical Computing).
Results
Search results and characteristics of included studies
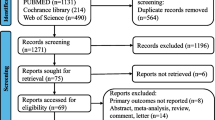
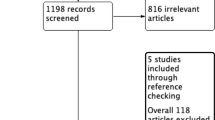
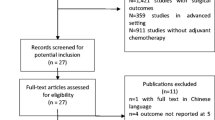
The selection was detailed in a PRISMA flow diagram (Fig. 1). A total of 1239 references were retrieved in our systematic search. After removing duplicate records and assessing the studies based on title and abstract, 1164 references were excluded and 75 full-text manuscripts were eligible and thoroughly reviewed for inclusion and exclusion criteria. Of these, 34 studies satisfied the eligibility criteria and formed the scope of the analysis, comprising 31,558 patients [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63].
A total of 31,558 patients were divided into 11,804 patients in the group ACT and 19,754 in the group No-ACT. The majority of the patients were on clinical stage II (13,378) with the median age ranging from 52.9 to 65.7 years. The follow-up ranged from 35 to 120 months. Study patient baseline characteristics of the included studies are summarized in Table 1.
Overall survival
OS was significantly prolonged in patients that received ACT versus No-ACT (HR 0.75; 95% CI 0.60–0.94; p = 0.015; I2 = 0%; Fig. 2A). The 5-year analysis also showed a statistically improved OS for the ACT group (OR 1.65; 95% CI 1.21–2.24; p = 0.001; I2 = 39%; Fig. 2B).
Disease-free survival
Analysis of DFS was based in 13 studies, which directly compared ACT versus No-ACT. A total of 1809 patients were included in the intervention group and 1927 in the control group. Figure 3A presents the following findings: (HR 0.94; 95% CI 0.76–1.17; p = 0.61; I2 = 17%; Fig. 3A). There was also no significant impact on DFS observed in the 5 years analysis (OR 1.19; 95% CI 0.82–1.74; p = 0.36; I2 = 43%; Fig. 3B).
Recurrence-free survival
RFS data were available for 11 studies; thus, 1703 and 1348 patients were included respectively in the experimental and control groups. The analysis, shown in Fig. 4A, yielded a (HR 1.10; 95% CI 0.87–1.40; p = 0.39; I2 = 0%). The 5-year RFS analysis showed no statistical significance (OR 1.08; 95% CI 0.78–1.51; p = 0.62; I2 = 0%; Fig. 4B).
Sensitivity analysis
We performed a leave-one-out sensitivity analysis for all outcomes. In general, heterogeneity was low in the majority of the outcomes assessed in this meta-analysis (I2 < 25%). Our overall analysis showed an increased heterogeneity in the 5-year OS and 5-year DFS outcomes (I2 = 43%). Despite conducting the sensitivity analysis, we were unable to identify the study responsible for the increased heterogeneity in OS. However, for DFS, by omitting Peng et al. [46], there was a substantial decrease in the heterogeneity for this outcome. The leave-one-out sensitivity analysis of the main outcomes is detailed in Supplementary Figure S1.
Estimation of publication bias
We conducted a funnel plot analysis for all outcomes. The X-axis corresponds to the odds ratio, while the Y-axis represents the standard error. The dashed lines indicate two standard errors on either side of the mean effect. Each circle is representative of one study. Test for asymmetry was statistically significant by Begg’s rank correlation between precision and effect size, and Egger’s regression test. In Fig. 5A, the symmetrical distribution of comparable studies in the funnel plot indicates that there is no evidence of publication bias in the outcomes comparing ACT versus No-ACT (Fig. 5A). Furthermore, the drapey plot result confirms the high reliability of our results (p = 0.01) (Fig. 5B). The funnel plot analysis of the main outcomes is detailed in Supplementary Figure S2.
Quality assessment
The individual assessment of each observational study included in the meta-analysis is depicted in Figure S3. All studies scored between 5 and 9 points. Overall, 13 of 34 studies were deemed at good quality. The studies by Peng et al. and Lichthardt et al. scored 5 points and were of poor quality due to not fulfilling the minimum criteria for outcome follow-up domains.
According to the GRADE assessment, the 5-year OS combined data from our 19 observational studies, the OS analysis combined 18 studies, while DFS was based on 13 studies, 5-year DFS and RFS on 11, and 5-year RFS was based on 9 studies. The GRADE quality assessment is detailed in the Supplementary Fig. 4.
Discussion
This systematic review and meta-analysis investigated the impact of adjuvant chemotherapy (ACT) versus no treatment (No-ACT) on overall survival (OS), disease-free survival (DFS), and recurrence-free survival (RFS) in patients with locally advanced rectal cancer after achieving complete pathological response (pCR) to neoadjuvant treatment. Our results demonstrate a substantial benefit for ACT in OS (HR 0.75; 95% CI 0.60–0.94; p = 0.015), indicating its ability to prolong survival compared to the control group. These findings are particularly encouraging, suggesting ACT as a potential therapeutic strategy for this patient group.
CR is a heterogeneous tumor type with a variety of possible treatments [64]. Most can be treated with surgery alone; however, a significant proportion of patients present with LARC require NAT with the aim of reducing tumor burden and increasing the effectiveness of the surgical procedure [65]. Understanding the clinical outcomes after pCR would help guide the precise selection of patients who would benefit from this intervention and drive the personalization of cancer treatment.
However, we observed no significant effect of ACT on DFS (HR 0.94; 95% CI 0.76–1.17; p = 0.61) or RFS (HR 1.10; 95% CI 0.87–1.40; p = 0.39). These results might imply that ACT may not directly prevent initial recurrence in patients with rectal cancer.
Several factors can explain the discrepancy between DFS and RFS with OS. ACT may act to eradicate micrometastases and residual disease after initial neoadjuvant treatment, contributing to the revitalization of the immune system and the control of molecular and biochemical mechanisms associated with initial disease progression [30, 66]. Thus, it is possible that these combined effects justify the benefit of this treatment for increased survival, even without preventing initial disease recurrence in treated patients.
Additionally, the inconclusive impact of ACT on DFS and RFS may be partially explained by the limitations of traditional follow-up methods. Studies have shown that some patients with early-stage disease experience delayed recurrences, which can only be captured through longer monitoring periods [12]. This highlights the need for extended follow-up, a need further emphasized by the emergence of circulating tumor DNA (ctDNA) as a promising tool.
Current methods for recurrence assessment in LARC, such as colonoscopy and carcinoembryonic antigen (CEA), have significant limitations. Colonoscopy, while offering high sensitivity and specificity for colorectal cancer diagnosis, can be met with patient resistance due to its invasive nature and potential complications [67,68,69]. CEA, the only biomarker recommended by the National Comprehensive Cancer Network for postoperative surveillance, suffers from insufficient sensitivity and specificity, limiting its effectiveness [70,71,72]. As a marker for residual micrometastases, ctDNA has recently demonstrated its ability to identify colon cancer patients who benefit from ACT based on postoperative ctDNA levels [73, 74]. While evidence in LARC remains preliminary, ctDNA holds promise for improved risk stratification and management in this patient population as well. Positive ctDNA status has been linked to a higher risk of recurrence after colorectal surgery [75,76,77,78]. This suggests that ctDNA-positive patients with LARC might benefit from intensified postoperative ACT regimens and more frequent follow-up to ensure timely detection and treatment of potential recurrences.
While adjuvant therapy remains to be fundamental in the treatment of CRC, recent research explores the potential of tumor-infiltrating lymphocytes (TILs) and the Immunoscore for not only predicting prognosis but also potentially informing treatment decisions beyond standard therapy [79]. The presence and density of TILs within the tumor microenvironment have been linked to patient survival in CRC. The meta-analyses have shown a significant association between high TILs and improved clinical outcomes such as OS, DFS, and cancer-specific survival (CSS) [80, 81]. This suggests that a robust anti-tumor immune response, as indicated by TIL infiltration, can positively influence patient prognosis. The immunoscore, a standardized approach that quantifies TIL density and distribution, has also been correlated with improved prognosis in CRC patients [79, 81]. Unlike adjuvant therapy, which directly targets cancer cells, TILs and immunoscore offer a prognostic tool. By assessing the pre-existing immune response within the tumor, these approaches can help predict a patient’s risk of recurrence after standard treatment. Similar to ctDNA analysis, high TILs and immunoscore could potentially guide decisions about follow-up intensity, ensuring which LARC patients would benefit from the use of ACT following NACT and surgery.
This meta-analysis has some limitations. First, this analysis is limited by the absence of well-designed, prospective randomized controlled trials evaluating the necessity of ACT for patients with rectal cancer achieving a pCR following neoadjuvant therapy and surgery. The included studies in this analysis were exclusively retrospective cohort studies, characterized by varying sample sizes, baseline characteristics, and treatment protocols. Consequently, the presence of information bias and confounding factors was unavoidable. Second, we were unable to perform a multivariate analysis and evaluate the effect of ACT in subgroups of patients with different disease stages, individual characteristics, and specific populations, which may limit the generalizability of our results. Third, the follow-up time was variable, potentially hindering the detection of significant differences in DFS and RFS. However, despite these limitations, robust results were obtained, indicating that ACT treatment has a potential benefit for OS. The low heterogeneity found in all outcomes: OS (I2 = 0%), DFS (I2 = 17%), and RFS (I2 = 0%), reinforces the reliability of the results found in this study. Future prospective long-term studies are needed to confirm and validate our findings.
Conclusion
In conclusion, this meta-analysis provides compelling evidence that adjuvant chemotherapy (ACT) improves overall survival in rectal cancer patients after complete pathological response, by uncertain mechanisms that are nor explained by improved disease specif survival or decreased cancer recurrence. Although ACT did not show a significant impact on disease-free survival and recurrence-free survival, the benefit in overall survival justifies the consideration of this therapeutic approach as part of the clinical management of these patients. Further research is needed to identify potential biomarkers and determine which patients would benefit from the use of adjvant chemotherapy following neoadjuvant and surgery.
Data availability
All data generated and/or analyzed during this study are included in this published article (and its supplementary information files).
Abbreviations
- CSS:
-
Cancer-specific survival
- CEA:
-
Carcinoembryonic antigen
- ctDNA:
-
Circulating tumor DNA
- CRC:
-
Colorectal cancer
- LARC:
-
Locally advanced rectal cancer
- NAT:
-
Neoadjuvant chemoradiotherapy
- TME:
-
Total mesorectal resection
- pCR:
-
Pathological complete response
- NCCN:
-
National Comprehensive Cancer Network
- ACT:
-
Adjuvant chemotherapy
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- RFS:
-
Recurrence-free survival
- CRC:
-
Colorectal cancer
- NOS:
-
Newcastle–Ottawa Scale
- HR:
-
Hazard ratio
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- TILS:
-
Tumor-infiltrating lymphocytes
- TNT:
-
Total neoadjuvant therapy
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- USA:
-
United States of America
References
Morgan E, Arnold M, Gini A et al (2023) Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 72(2):338–344
Stoffel EM, Murphy CC (2020) Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology 158(2):341–353. https://doi.org/10.1053/j.gastro.2019.07.055
Cheong C, Oh SY, Kim YB et al (2019) Differences in biological behaviors between young and elderly patients with colorectal cancer. PLoS ONE 14(6):e0218604. https://doi.org/10.1371/journal.pone.0218604
Ryan ÉJ, Creavin B, Sheahan K (2020) Delivery of personalized care for locally advanced rectal cancer: incorporating pathological, molecular genetic, and immunological biomarkers into the multimodal paradigm. Front Oncol 10:1369
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, Van Krieken JH et al (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Sauer R, Becker H, Hohenberger W et al (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17):1731–1740
Chalabi M, Fanchi LF, Dijkstra KK et al (2020) Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 26:566–576
Cercek A, Lumish M, Sinopoli J et al (2022) PD-1 blockade in mismatch repair–deficient, locally advanced rectal cancer. N Engl J Med 386:2363–2376
Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U (2005) Swedish rectal cancer trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 23:5644–5650
van der Valk MJM, Hilling DE, Bastiaannet E et al (2018) Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 391(10139):2537–2545
Kim HG, Kim HS, Yang SY, Han YD, Cho MS, Hur H, Min BS, Lee KY, Kim NK (2021) Early recurrence after neoadjuvant chemoradiation therapy for locally advanced rectal cancer: characteristics and risk factors. Asian J Surg 44(1):298–302
Alexandrescu ST, Dumitru AV, Babiuc RD, Costea RV (2021) Assessment of clinical and pathological complete response after neoadjuvant chemoradiotherapy in rectal adenocarcinoma and its therapeutic implications. Rom J Morphol Embryol 62(2):411–425
de Campos-Lobato LF, Stocchi L, da Luz MA, Geisler D, Dietz DW, Lavery IC et al (2011) Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol 18(6):1590–1598
Belluco C, De Paoli A, Canzonieri V, Sigon R, Fornasarig M, Buonadonna A et al (2011) Long-term outcome of patients with complete pathologic response after neoadjuvant chemoradiation for cT3 rectal cancer: implications for local excision surgical strategies. Ann Surg Oncol 18(13):3686–3693. https://doi.org/10.1245/s10434-011-1822-0
Wasmuth HH, Rekstad LC, Tranø G (2016) The outcome and the frequency of pathological complete response after neoadjuvant radiotherapy in curative resections for advanced rectal cancer: a population-based study. Colorectal Dis 18(1):67–72
Zorcolo L, Rosman AS, Restivo A et al (2012) Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol 19(9):2822–2832
Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, McCormick J, Kirichenko A (2020) Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer: a National Cancer Database (NCDB) analysis. Ann Surg 271(4):716–723
Benson AB, Venook AP, Al-Hawary MM et al (2022) Rectal Cancer, Version 2202, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 20(10):1139–67
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Definition of overall survival - NCI Dictionary of Cancer Terms-NCI [Internet]. 2011 [cited 2024 May 15]. Data available: 2024 May 09. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/overall-survival
Definition of disease-free survival - NCI Dictionary of Cancer Terms-NCI [Internet]. 2011 [cited 2024 May 16]. Data available: 2024 May 09. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/disease-free-survival
Definition of relapse-free survival - NCI Dictionary of Cancer Terms-NCI [Internet]. 2011 [cited 2024 May 16]. Data available: 2024 May 09. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/relapse-free-survival
Ottawa Hospital Research Institute. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 20 Feb 2023.
Chapter 13: Assessing risk of bias due to missing results in a synthesis | Cochrane training. https://training.cochrane.org/handbook/current/chapter-13. Accessed 01 Feb 2023.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S et al (2011) GRADE Guidelines: 3. Rating the Quality of Evidence. J Clin Epidemiol 64:401–406
Higgins JPT (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
IntHout J, Ioannidis JPA, Borm GF (2014) The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 14:25
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Chen M, Zhang J, Hou Y et al (2023) Clinical significance of adjuvant chemotherapy for pathological complete response rectal cancer patients with acellular mucin pools after neoadjuvant chemoradiotherapy. Therap Adv Gastroenterol 16:17562848221117876
Lai SH, Vogel JD, Vemuru S et al (2023) Improved survival after adjuvant therapy in locally advanced rectal cancer patients with pathologic complete response. Dis Colon Rectum 66(7):983–993
Bliggenstorfer JT, Ginesi M, Steinhagen E, Stein SL (2022) Lymph node yield after rectal resection is a predictor of survival among patients with node negative rectal adenocarcinoma. Surgery 172(5):1292–9.27
Fukui Y, Hida K, Hoshino N et al (2022) Oncologic benefit of adjuvant chemotherapy for locally advanced rectal cancer after neoadjuvant chemoradiotherapy and curative surgery with selective lateral pelvic lymph node dissection: an international retrospective cohort study. Eur J Surg Oncol 48(7):1631–7.28
Kuo YH, Lin YT, Ho CH et al (2022) Adjuvant chemotherapy and survival outcomes in rectal cancer patients with good response (ypT0-2N0) after neoadjuvant chemoradiotherapy and surgery: a retrospective nationwide analysis. Front Oncol 12(1087778):29
Nafouje SA, Liu YJ, Kamarajah SK, Salti GI, Dahdaleh F (2022) Adjuvant chemotherapy after neoadjuvant chemoradiation and proctectomy improves survival irrespective of pathologic response in rectal adenocarcinoma: a population-based cohort stud. Int J Colorectal Dis 37(10):2137–2148
Aquino de Moraes FC, Dantas Leite Pessôa FD, Duarte de Castro Ribeiro CH et al (2024) Trifluridine-tipiracil plus bevacizumab versus trifluridine-tipiracil monotherapy for chemorefractory metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer 24:674. https://doi.org/10.1186/s12885-024-12447-8
Jiang T, Liu S, Wu X et al (2021) Nomogram to predict distant metastasis probability for pathological complete response rectal cancer patients after neoadjuvant chemoradiotherapy. Cancer Manag Res 13:4751–4761
He F, Ju HQ, Ding Y et al (2020) Association between adjuvant chemotherapy and survival in patients with rectal cancer and pathological complete response after neoadjuvant chemoradiotherapy and resection. Br J Cancer 123(8):1244–1252
Capirci C, Valentini V, Cionini L et al (2008) Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys 72(1):99–107
Gahagan JV, Whealon MD, Phelan MJ et al (2020) Improved survival with adjuvant chemotherapy in locally advanced rectal cancer patients treated with preoperative chemoradiation regardless of pathologic response. Surg Oncol 32:35–40
Voss RK, Lin JC, Roper MT et al (2020) Adjuvant chemotherapy does not improve recurrence-free survival in patients with stage 2 or stage 3 rectal cancer after neoadjuvant chemoradiotherapy and total mesorectal excision. Dis Colon Rectum 63(4):427–440
Hu X, Li YQ, Ma XJ, Zhang L, Cai SJ, Peng JJ (2019) Adjuvant chemotherapy for rectal cancer with complete pathological response (pCR) may not be necessary: a pooled analysis of 5491 patients. Cancer Cell Int 19:127
Nguyen A, James DR, Dozois EJ, Kelley SR, Mathis KL (2019) The role of adjuvant chemotherapy in ypT0N0 rectal adenocarcinoma. J Gastrointest Surg 23(11):2263–2268
Dossa F, Acuna SA, Rickles AS et al (2018) Association between adjuvant chemotherapy and overall survival in patients with rectal cancer and pathological complete response after neoadjuvant chemotherapy and resection. JAMA Oncol 4(7):930–937
Lu Z, Cheng P, Zhang MG, Wang XS, Zheng ZX (2018) Is adjuvant chemotherapy necessary for patients with ypT0-2N0 rectal cancer treated with neoadjuvant chemoradiotherapy and curative surgery? Gastroenterol Rep (Oxf ) 6(4):277–283
Peng JH, Lin JZ, Rong YM et al (2018) Oxaliplatin-containing adjuvant chemotherapy improves the survival of locally advanced rectal cancer patients with pathological complete response after pre-operative chemoradiotherapy. Gastroenterol Rep (Oxf ) 6(3):195–201
Polanco PM, Mokdad AA, Zhu H, Choti MA, Huerta S (2018) Association of adjuvant chemotherapy with overall survival in patients with rectal cancer and pathologic complete response following neoadjuvant chemotherapy and resection. JAMA Oncol 4(7):938–943
Turner MC, Keenan JE, Rushing CN et al (2019) Adjuvant chemotherapy improves survival following resection of locally advanced rectal cancer with pathologic complete response. J Gastrointest Surg 23(8):1614–1622
Gamaleldin M, Church JM, Stocchi L, Kalady M, Liska D, Gorgun E (2017) Is routine use of adjuvant chemotherapy for rectal cancer with complete pathological response justified? Am J Surg 213(3):478–483
Lichthardt S, Zenorini L, Wagner J et al (2017) Impact of adjuvant chemotherapy after neoadjuvant radio- or radiochemotherapy for patients with locally advanced rectal cancer. J Cancer Res Clin Oncol 143(11):2363–2373
Lorenzon L, Parini D, Rega D et al (2017) Long-term outcomes in ypT0 rectal cancers: an international multi-centric investigation on behalf of Italian Society of Surgical Oncology Young Board (YSICO). Eur J Surg Oncol 43(8):1472–1480
Shahab D, Gabriel E, Attwood K et al (2017) Adjuvant chemotherapy is associated with improved overall survival in locally advanced rectal cancer after achievement of a pathologic complete response to chemoradiation. Clin Colorectal Cancer 16(4):300–307
Kim CG, Ahn JB, Shin SJ et al (2017) Role of adjuvant chemotherapy in locally advanced rectal cancer with ypT0-3N0 after preoperative chemoradiation therapy and surgery. BMC Cancer 17(1):615
Kuan FC, Lai CH, Ku HY et al (2017) The survival impact of delayed surgery and adjuvant chemotherapy on stage II/III rectal cancer with pathological complete response after neoadjuvant chemoradiation. Int J Cancer 140(7):1662–1669
Tay RY, Jamnagerwalla M, Steel M et al (2017) Survival impact of adjuvant chemotherapy for resected locally advanced rectal adenocarcinoma. Clin Colorectal Cancer 16(2):e45-54
Xu Z, Mohile SG, Tejani MA et al (2017) Poor compliance with adjuvant chemotherapy use associated with poorer survival in patients with rectal cancer: an NCDB analysis. Cancer 123(1):52–61
Zhou J, Qiu H, Lin G et al (2016) Is adjuvant chemotherapy necessary for patients with pathological complete response after neoadjuvant chemoradiotherapy and radical surgery in locally advanced rectal cancer? Long-term analysis of 40 ypCR patients at a single center. Int J Colorectal Dis 31(6):1163–1168
Lee KH, Kim JC, Kim JY, Kim JS (2015) Oncologic results and prognostic predictors of patients with locally advanced rectal cancer showing ypN0 after radical surgery following neoadjuvant chemoradiotherapy. Int J Colorectal Dis 30(8):1041–1050
Maas M, Nelemans PJ, Valentini V et al (2015) Adjuvant chemotherapy in rectal cancer: defining subgroups who may benefit after neoadjuvant chemoradiation and resection: a pooled analysis of 3,313 patients. Int J Cancer 137(1):212–220
Geva R, Itzkovich E, Shamai S et al (2014) Is there a role for adjuvant chemotherapy in pathological complete response rectal cancer tumors following neoadjuvant chemoradiotherapy? J Cancer Res Clin Oncol 140(9):1489–1494
Kiran RP, Kirat HT, Burgess AN, Nisar PJ, Kalady MF, Lavery IC (2012) Is adjuvant chemotherapy really needed after curative surgery for rectal cancer patients who are node-negative after neoadjuvant chemoradiotherapy? Ann Surg Oncol 19(4):1206–1212
Govindarajan A, Reidy D, Weiser MR et al (2011) Recurrence rates and prognostic factors in ypN0 rectal cancer after neoadjuvant chemoradiation and total mesorectal excision. Ann Surg Oncol 18(13):3666–3672
Yeo SG, Kim DY, Kim TH et al (2010) Pathologic complete response of primary tumor following preoperative chemoradiotherapy for locally advanced rectal cancer: long-term outcomes and prognostic significance of pathologic nodal status (KROG 09–01). Ann Surg 252(6):998–1004
Ahmad R, Singh JK, Wunnava A, Al-Obeed O, Abdulla M, Srivastava SK (2021) Emerging trends in colorectal cancer: Dysregulated signaling pathways (Review). Int J Mol Med 47(3):14
Daprà V, Airoldi M, Bartolini M et al (2023) Total neoadjuvant treatment for locally advanced rectal cancer patients: where do we stand? Int J Mol Sci 24(15):12159
Baloyiannis I, Perivoliotis K, Vederaki S, Koukoulis G, Symeonidis D, Tzovaras G (2021) Current evidence regarding the role of adjuvant chemotherapy in rectal cancer patients with pathologic complete response after neoadjuvant chemoradiotherapy: a systematic review and meta-analysis. Int J Colorectal Dis 36(7):1395–1406
Barnett S, Hung A, Tsao R et al (2016) Capnographic monitoring of moderate sedation during low-risk screening colonoscopy does not improve safety or patient satisfaction: a prospective cohort study. Am J Gastroenterol 111(3):388–394
Ladabaum U, Mannalithara A, Meester RGS, Gupta S, Schoen RE (2019) Cost-effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 years. Gastroenterology 157(1):137–148
Yeoh A, Mannalithara A, Ladabaum U (2022) Cost-effectiveness of earlier or more intensive colorectal cancer screening in overweight and obese patients. Clin Gastroenterol Hepatol 21:507–519
Shinkins B, Nicholson BD, Primrose J et al (2017) The diagnostic accuracy of a single CEA blood test in detecting colorectal cancer recurrence: results from the FACS trial. PLoS ONE 12(3):e0171810
Su BB, Shi H, Wan J (2012) Role of serum carcinoembryonic antigen in the detection of colorectal cancer before and after surgical resection. World J Gastroenterol 18(17):2121–2126
Sveen A, Kopetz S, Lothe RA (2020) Biomarker-guided therapy for colorectal cancer: strength in complexity. Nat Rev Clin Oncol 17(1):11–32
Dizdarevic E, Hansen TF, Jakobsen A. The prognostic importance of ctDNA in rectal cancer: a critical reappraisal. cancers (Basel). 2022;14(9):2252. Published 2022 Apr 30. https://doi.org/10.3390/cancers14092252
Kotani D, Oki E, Nakamura Y et al (2023) Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med 29:127–134
Tie J, Cohen JD, Wang Y et al (2019) Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol 5(12):1710–1717
Tie J, Wang Y, Tomasetti C et al (2016) Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med 8(346):346ra392
Wang Y, Li L, Cohen JD et al (2019) Prognostic potential of circulating tumor DNA measurement in postoperative surveillance of nonmetastatic colorectal cancer. JAMA Oncol 5(8):1118–1123
de Moraes FCA, Pessôa FDDL et al (2024) Trifluridine-tipiracil plus bevacizumab versus trifluridine-tipiracil monotherapy for chemorefractory metastatic colorectal cancer: a systematic review and meta-analysis. BMC Cancer 24(1):674
Pagès F, Mlecnik B, Marliot F et al (2018) International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391(10135):2128–2139
Idos GE, Kwok J, Bonthala N, Kysh L, Gruber SB, Qu C (2020) The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: a systematic review and meta-analysis. Sci Rep 10(1):3360
Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G (2017) The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol 14(12):717–734
Acknowledgements
We thank the Federal University of Pará (UFPA) and the Center for Research in Oncology (NPO/UFPA). The design of the study, sample collection, data analysis, interpretation, and manuscript writing were conducted independently of any influence or involvement from fundings agencies.
Funding
This research was funded by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Pró-Reitoria de Pesquisa e Pós-Graduação da UFPA (PROPESP).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. F.C.A.M. conceived the project, and material preparation, data collection, and analysis were performed by F.C.A.M., R.M.R.B., F.A.K., and M.E.C.S. The figures and tables were created by F.C.A.M., R.M.R.B., F.A.K., and M.E.C.S. The first draft of the manuscript was written by F.C.A.M., R.M.R.B., F.A.K., and M.E.C.S., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Moraes, F.C.A., Kelly, F.A., Souza, M.E.C. et al. Impact of adjuvant chemotherapy on survival after pathological complete response in rectal cancer: a meta-analysis of 31,558 patients. Int J Colorectal Dis 39, 96 (2024). https://doi.org/10.1007/s00384-024-04668-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-024-04668-x