Abstract
Background
Melanosis coli is characterized by brown mucosa with pigmentation. Studies have showed an increased adenoma detection rate in melanosis patients, whether it is caused by a contrast effect or an oncogenic effect is still controversial. The detection of serrated polys in melanosis patients remains unknown.
Aims
The study aimed to clarify the correlation of adenoma detection rate with melanosis coli and discuss outcomes in less-experienced endoscopists. Serrated polyp detection rate was also been investigated.
Methods
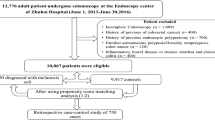
A total of 2150 patients and 39,630 controls were enrolled. A propensity score matching method was used to balance covariates between the two groups. The detection of polyps, adenomas, serrated polyps, and their features was analyzed.
Results
The polyp detection rate (44.65% vs 41.01%, P = 0.005) and adenoma detection rate (30.34% vs 23.92%, P < 0.001) were significantly higher, and the serrated polyp detection rate (0.93% vs 1.58%, P = 0.033) was significantly lower in melanosis coli. The percentage of low-risk adenomas (44.60% vs 39.16%, P < 0.001) and polyps with 6 to 10 mm in size (20.16% vs 16.21%, P < 0.001) were higher in melanosis coli. The detection of large serrated polyps was lower (0.11% vs 0.41%, P = 0.026) in melanosis coli.
Conclusion
Melanosis coli correlates with an increased adenoma detection rate. The detection of large serrated polyps was lower in melanosis patients. Melanosis coli may not be considered a precancerous lesion.



Similar content being viewed by others
Data availability
The datasets underlying the current study will be available by the corresponding author for reasonable request.
References
van Gorkom BA, Karrenbeld A, van Der Sluis T, Koudstaal J, de Vries EG, Kleibeuker JH (2000) Influence of a highly purified senna extract on colonic epithelium. Digestion 61(2):113–120. https://doi.org/10.1159/000007743
Mellouki I, Meyiz H (2013) Melanosis coli: a rarity in digestive endoscopy. Pan Afr Med J 16:86. https://doi.org/10.11604/pamj.2013.16.86.3331
de Witte P (1993) Metabolism and pharmacokinetics of anthranoids. Pharmacology 47(Suppl 1):86–97. https://doi.org/10.1159/000139847
Renda A, Vergani C, Venturi M, Ferrero S, Del Gobbo A (2018) Diffuse melanosis in pericolic lymph nodes associated with laxative abuse and colorectal cancer. Int J Surg Pathol 26(1):37–38. https://doi.org/10.1177/1066896917718346
van Gorkom BA, de Vries EG, Karrenbeld A, Kleibeuker JH (1999) Review article: anthranoid laxatives and their potential carcinogenic effects. Aliment Pharmacol Ther 13(4):443–452. https://doi.org/10.1046/j.1365-2036.1999.00468.x
Willems M, van Buuren HR, de Krijger R (2003) Anthranoid self-medication causing rapid development of melanosis coli. Neth J Med 61(1):22–24
Grilo I, Torres-Gómez J, Gómez-Regife L (2014) Atypical melanosis coli resembling the appearance of cheetah skin. Endoscopy 46(Suppl 1):UCTN:E437-8. https://doi.org/10.1055/s-0034-1377427
Malik AH, Andrabi SI, Niayesh M (2008) Pseudo-obstruction with pitch black colon–a very rare presentation of melanosis coli. Ulst Med J 77(1):54–55
Lombardi N, Crescioli G, Maggini V, Bellezza R, Landi I, Bettiol A et al (2022) Anthraquinone laxatives use and colorectal cancer: a systematic review and meta-analysis of observational studies. Phytother Res 36(3):1093–1102. https://doi.org/10.1002/ptr.7373
Zhang Y, Zhan TT, Dong ZY, Sun HH, Wang JW, Chen Y et al (2022) Melanosis coli: A factor not associated with histological progression of colorectal polyps. J Dig Dis 23(5–6):302–309. https://doi.org/10.1111/1751-2980.13100
Blackett JW, Rosenberg R, Mahadev S, Green PHR, Lebwohl B (2018) Adenoma detection is increased in the setting of melanosis coli. J Clin Gastroenterol 52(4):313–318. https://doi.org/10.1097/mcg.0000000000000756
Kassim SA, Abbas M, Tang W, Wu S, Meng Q, Zhang C et al (2020) Retrospective study on melanosis coli as risk factor of colorectal neoplasm: a 3-year colonoscopic finding in Zhuhai Hospital, China. Int J Colorectal Dis 35(2):213–222. https://doi.org/10.1007/s00384-019-03435-7
Liu ZH, Foo DCC, Law WL, Chan FSY, Fan JKM, Peng JS (2017) Melanosis coli: harmless pigmentation? A case-control retrospective study of 657 cases. PLoS ONE 12(10):e0186668. https://doi.org/10.1371/journal.pone.0186668
Nusko G, Schneider B, Ernst H, Wittekind C, Hahn EG (1997) Melanosis coli–a harmless pigmentation or a precancerous condition? Z Gastroenterol 35(5):313–318
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system 4th[M]. Lyon: IARC Press
Gupta S, Lieberman D, Anderson J, Burke C, Dominitz J, Kaltenbach T et al (2020) Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US multi-society task force on colorectal cancer. Gastroenterology 158(4):1131–53.e5. https://doi.org/10.1053/j.gastro.2019.10.026
Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S et al (2020) Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – Update 2020. Endoscopy 52(8):687–700. https://doi.org/10.1055/a-1185-3109
Kaminski M, Thomas-Gibson S, Bugajski M, Bretthauer M, Rees C, Dekker E et al (2017) Performance measures for lower gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 49(3):378–397. https://doi.org/10.1055/s-0043-103411
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Leggett B, Whitehall V (2010) Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 138(6):2088–2100. https://doi.org/10.1053/j.gastro.2009.12.066
Siegers CP, Hertzberg-Lottin EV, Otte M, Schneider B (1993) Anthranoid laxative abuse–a risk for colorectal cancer? Gut 34(8):1099–1101. https://doi.org/10.1136/gut.34.8.1099
Nusko G, Schneider B, Schneider I, Wittekind C, Hahn EG (2000) Anthranoid laxative use is not a risk factor for colorectal neoplasia: results of a prospective case control study. Gut 46(5):651–655. https://doi.org/10.1136/gut.46.5.651
Katsumata R, Manabe N, Fujita M, Ayaki M, Sunago A, Kamada T, Monobe Y, Kawamoto H, Haruma K (2021) Colorectal neoplasms in melanosis coli: a survey in Japan and a worldwide meta-analysis. Int J Color Dis. https://doi.org/10.1007/s00384-021-03970-2
Brown SR, Baraza W, Din S, Riley S (2016) Chromoscopy versus conventional endoscopy for the detection of polyps in the colon and rectum. Cochrane Database Syst Rev 4(4):CD006439. https://doi.org/10.1002/14651858.CD006439.pub4
Brooker JC, Saunders BP, Shah SG, Thapar CJ, Thomas HJ, Atkin WS et al (2002) Total colonic dye-spray increases the detection of diminutive adenomas during routine colonoscopy: a randomized controlled trial. Gastrointest Endosc 56(3):333–338. https://doi.org/10.1016/s0016-5107(02)70034-5
Hurlstone DP, Cross SS, Slater R, Sanders DS, Brown S (2004) Detecting diminutive colorectal lesions at colonoscopy: a randomised controlled trial of pan-colonic versus targeted chromoscopy. Gut 53(3):376–380. https://doi.org/10.1136/gut.2003.029868
Omata F, Ohde S, Deshpande GA, Kobayashi D, Masuda K, Fukui T (2014) Image-enhanced, chromo, and cap-assisted colonoscopy for improving adenoma/neoplasia detection rate: a systematic review and meta-analysis. Scand J Gastroenterol 49(2):222–237. https://doi.org/10.3109/00365521.2013.863964
Hetzel JT, Huang CS, Coukos JA, Omstead K, Cerda SR, Yang S et al (2010) Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 105(12):2656–2664. https://doi.org/10.1038/ajg.2010.315
Payne SR, Church TR, Wandell M, Rösch T, Osborn N, Snover D et al (2014) Endoscopic detection of proximal serrated lesions and pathologic identification of sessile serrated adenomas/polyps vary on the basis of center. Clin Gastroenterol Hepatol 12(7):1119–1126. https://doi.org/10.1016/j.cgh.2013.11.034
Álvarez C, Andreu M, Castells A, Quintero E, Bujanda L, Cubiella J et al (2013) Relationship of colonoscopy-detected serrated polyps with synchronous advanced neoplasia in average-risk individuals. Gastrointest Endosc 78(2):333–41.e1. https://doi.org/10.1016/j.gie.2013.03.003
Anderson JC, Butterly LF, Goodrich M, Robinson CM, Weiss JE (2013) Differences in detection rates of adenomas and serrated polyps in screening versus surveillance colonoscopies, based on the new hampshire colonoscopy registry. Clin Gastroenterol Hepatol 11(10):1308–1312. https://doi.org/10.1016/j.cgh.2013.04.042
Verheyen E, Castaneda D, Gross SA, Popov V (2021) Increased sessile serrated adenoma detection rate with mechanical new technology devices: a systematic review and meta-analysis. J Clin Gastroenterol 55(4):335–342. https://doi.org/10.1097/mcg.0000000000001363
East JE, Atkin WS, Bateman AC, Clark SK, Dolwani S, Ket SN et al (2017) British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 66(7):1181–1196. https://doi.org/10.1136/gutjnl-2017-314005
Kumbhari V, Behary J, Hui JM (2013) Prevalence of adenomas and sessile serrated adenomas in Chinese compared with Caucasians. J Gastroenterol Hepatol 28(4):608–612. https://doi.org/10.1111/jgh.12100
Chang MC, Ma CC, Yu HC, Hsu PI, Liao JB, Huang CC (2020) Detection and clinical characteristics of serrated polyps and conventional adenomas between patients in the outpatient and physical checkup unit receiving colonoscopy. Int J Colorectal Dis 35(11):1979–1987. https://doi.org/10.1007/s00384-020-03665-0
Cao HL, Chen X, Du SC, Song WJ, Wang WQ, Xu MQ et al (2016) Detection rate, distribution, clinical and pathological features of colorectal serrated polyps. Chin Med J 129(20):2427–2433. https://doi.org/10.4103/0366-6999.191759
Chen S, Sun K, Chao K, Sun Y, Hong L, Weng Z et al (2018) Detection rate and proximal shift tendency of adenomas and serrated polyps: a retrospective study of 62,560 colonoscopies. Int J Colorectal Dis 33(2):131–139. https://doi.org/10.1007/s00384-017-2951-0
Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW et al (2012) Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 107(9):1315–1330. https://doi.org/10.1038/ajg.2012.161
van Toledo D, IJspeert JEG, Dekker E (2022) Current approaches in managing colonic serrated polyps and serrated polyposis. Annu Rev Med 73:293–306. https://doi.org/10.1146/annurev-med-042220-024703
van Toledo D, IJspeert JEG, Bossuyt PMM, Bleijenberg AGC, van Leerdam ME, van der Vlugt M et al (2022) Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol. 7(8):747–754. https://doi.org/10.1016/s2468-1253(22)00090-5
Spadaccini M, Iannone A, Maselli R, Badalamenti M, Desai M, Chandrasekar VT et al (2021) Computer-aided detection versus advanced imaging for detection of colorectal neoplasia: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 6(10):793–802. https://doi.org/10.1016/s2468-1253(21)00215-6
Aziz M, Desai M, Hassan S, Fatima R, Dasari CS, Chandrasekar VT et al (2019) Improving serrated adenoma detection rate in the colon by electronic chromoendoscopy and distal attachment: systematic review and meta-analysis. Gastrointest Endosc 90(5):721–31.e1. https://doi.org/10.1016/j.gie.2019.06.041
He X, Hang D, Wu K, Nayor J, Drew DA, Giovannucci EL et al (2020) Long-term risk of colorectal cancer after removal of conventional adenomas and serrated polyps. Gastroenterology 158(4):852–61.e4. https://doi.org/10.1053/j.gastro.2019.06.039
Symonds E, Anwar S, Young G, Meng R, Coats M, Simpson K et al (2019) Sessile serrated polyps with synchronous conventional adenomas increase risk of future advanced neoplasia. Dig Dis Sci 64(6):1680–1685. https://doi.org/10.1007/s10620-019-5454-8
Katsumata R, Manabe N, Monobe Y, Tanikawa T, Ayaki M, Suehiro M et al (2022) Severe grade of melanosis coli is associated with a higher detection rate of colorectal adenoma. J Clin Biochem Nutr. 71(2):165–171. https://doi.org/10.3164/jcbn.22-19
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yan Wang, Xiaotong Niu and Fei Gao. The first draft of the manuscript was written by Yan Wang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the Institution Review Board of Chinese PLA General hospital.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Li, L., Niu, X. et al. Melanosis coli: a contrast effect or an oncogenic effect? A large-scale retrospective cohort study. Int J Colorectal Dis 38, 63 (2023). https://doi.org/10.1007/s00384-023-04357-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-023-04357-1