Abstract
Purpose
In the treatment of early-stage rectal cancer, a growing number of studies have shown that transanal endoscopic microsurgery is one of the alternatives to radical surgery adhering to total mesorectal excision that can reduce the incidence of adverse events without compromising treatment outcomes. The purpose of this meta-analysis is to compare the safety and treatment effect of transanal endoscopic microsurgery and radical surgery adhering to total mesorectal excision to provide a basis for clinical treatment selections.
Method
We searched the literatures of four major databases, PubMed, Embase, Web of science, and Cochrane Library, without limitation of time. The literatures included randomized controlled studies and cohort studies comparing two surgical procedures of transanal endoscopic microsurgery and radical surgery adhering to total mesorectal excision. Treatment effectiveness and safety results of transanal endoscopic microsurgery and radical surgery were extracted from the included literatures and statistically analyzed using RevMan5.4 and stata17.
Result
Ultimately, 13 papers were included in the study including 5 randomized controlled studies and 8 cohort studies. The results of the meta-analysis showed that the treatment effect and safety of both transanal endoscopic microsurgery and radical surgery in distant metastasis (RR, 0.59 (0.34, 1.02), P > 0.05), overall recurrence (RR, 1.49 (0.96, 2.31), P > 0.05), disease-specific-survival (RR, 0.74 (0.09, 1.57), P > 0.05), dehiscence of the sutureline or anastomosis leakage (RR, 0.57 (0.30, 1.06), P > 0.05), postoperative bleeding (RR, 0.47 (0.22, 0.99), P > 0.05), and pneumonia (RR, 0.37, (0.10, 1.40), P > 0.05) were not significantly different. However, they differ significantly in perioperative mortality (RR, 0.26 (0.07, 0.93, P < 0.05)), local recurrence (RR, 2.51 (1.53, 4.21), P < 0.05),_overall survival_ (RR, 0.88 (0.74, 1.00), P < 0.05), disease-free-survival (RR, 1.08 (0.97, 1.19), P < 0.05), temporary stoma (RR, 0.05 (0.01, 0.20), P < 0.05), permanent stoma (RR, 0.16 (0.08, 0.33), P < 0.05), postoperative complications (RR, 0.35 (0.21, 0.59), P < 0.05), rectal pain (RR, 1.47 (1.11, 1.95), P < 0.05), operation time (RR, −97.14 (−115.81, −78.47), P < 0.05), blood loss (RR, −315.52 (−472.47, −158.57), P < 0.05), and time of hospitalization (RR, −8.82 (−10.38, −7.26), P < 0.05).
Conclusion
Transanal endoscopic microsurgery seems to be one of the alternatives to radical surgery for early-stage rectal cancer, but more high-quality clinical studies are needed to provide a reliable basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, colorectal cancer ranks third and second in terms of incidence and mortality of all tumors, respectively, posing a serious threat to human life and health [1].
With the improvement of health awareness and medical treatment, an incraeasing number of patients of rectal cancer can be diagnosed at early stage, but the choice of surgical procedure for early rectal cancer is still controversial. Radical surgery following the principles of total mesorectal excision has been the standard procedure for early-stage rectal cancer, which was promoted by professor Richard (Bill) Heald and is being embraced by more clinicians and patients. The scope of total mesorectal excision includes the rectum, rectal mesentery, and occult metastatic lymph nodes. Five-year cancer-specific survival is expected to exceed 95% [2, 3]. Although radical surgery adhering to total mesorectal excision is effective in removing tumors and reducing tumor recurrence rate, there is still a 2% mortality rate and a significant risk of local recurrence [4, 5]. At the same time, postoperative complications such as bowel dysfunction, urinary incontinence, sexual dysfunction, and temporary or permanent stoma occur due to excessive surgical excision, which seriously impairs the quality of life [6,7,8]. Therefore, with the development of medical technology, transanal endoscopic microsurgery has received more attention. In 1983, Buess et al. introduced transanal endoscopic microsurgery, which is usually used for T1 or T2 without lymphovascular invasion and distant metastasis. Transanal endoscopic microsurgery (TEM) is an advanced surgical procedure performed through the anus to remove polyps and early cancers from the rectum. The surgery is minimally invasive. The advantages of transanal endoscopic microsurgery include shorter hospitalization time, less intraoperative bleeding, shorter operative time, higher rectum preservation rate, preserving anal function, lower perioperative mortality rate, and lower postoperative complication rate [9,10,11]. Transanal endoscopic surgery has been shown to be more effective in terms of reducing the incidence of postoperative complications. In early-stage rectal cancer, the probability of lymph node metastasis is 8.6% [9]. However, transanal endoscopic microsurgery cannot remove occult metastatic lymph nodes and may obtain positive margins, which in turn leads to a higher rate of local recurrence [12].
Because of the advantages and disadvantages of both surgical approaches, there is uncertainty in the choice of surgical approach for patients with early-stage rectal cancer. Therefore, the purpose of this meta-analysis is to compare the safety and short-term or long-term outcomes of the two surgical approaches to provide a basis for treatment decisions in clinical practice Figs. 1, 2 and 3.
Methods
Search strategy and study selection
To conduct this meta-analysis, we searched PubMed, Embase, Web of Science, and Cochrane Library four major English databases, with language of the publications limited to English and publication time unrestricted and with a combination of subject terms and free words to develop a search strategy; in order to obtain all literatures related to the selected topic, the search strategy is shown in Table 1. Two researchers independently read the titles and abstracts of the retrieved literatures. Duplicate studies, conference abstracts, reviews, animal experiments, inconsistent interventions, and irrelevant research content have been excluded. And randomized controlled studies and cohort studies including interventions for patients of early rectal cancer with TEM and radical surgery adhering to total mesorectal excision have been included. After the initial screening, the full text was read to determine the included literature. Those which could not access the full text would also be excluded. Finally, the two investigators reached a consensus through discussion to determine the included literature. The two investigators extracted the perioperative indicators and tumor outcomes after the two interventions of TEM and RS, radical surgery adhering to total mesorectal excision.
Inclusion and exclusion criteria
Inclusion criteria
The inclusion criteria were as follows:
Exclusion criteria
The exclusion criteria were as follows:
-
1.
Patients with non-rectal cancer or higher stage of rectal cancer (UICC II–IV)
-
2.
Any other interventions or comparators or absence of interventions
-
3.
Literature in other languages
-
4.
Lack of sufficient data or results
-
5.
Duplicate publications
-
6.
In vitro experiments, animal experiments, non-comparative studies, reviews, letters, guidelines, case reports, pathological mechanisms, conference abstracts, expert opinions, editorials, and comments
-
7.
Full text not available
Data extraction
According to the inclusion and exclusion criteria, two reviewers independently extracted relevant data from the included publications using a standard data extraction form, and the final extraction results were determined through discussions. The outcome measures extracted from the literatures were as follows:
Study characteristics
The study characteristics were as follows: first author, year of publication, country, type of trial, study period, and sample size.
Patient baseline
The patient baseline was as follows: intervention mode, preoperative T stage, preoperative therapy, age (year old), gender (male/female), R0 resection (%), histological grade, distance from the anus, lymph node metastasis, and follow-up time.
Study outcomes
The study outcomes were the following: operation time, blood loss, time of hospitalization, permanent stoma, temporary stoma, postoperative complications, postoperative bleeding, anastomosis stenosis, dehiscence of the suture line or anastomosis leakage, pneumonia, and rectal pain.
Follow-up outcomes
The follow-up outcomes were local recurrence, distant metastasis, overall recurrence, disease-free survival, disease-specific survival, perioperative mortality, and overall survival.
Quality assessment
Two investigators used the Newcastle–Ottawa scale (NOS) [13] to independently evaluate cohort studies. The NOS evaluation scale consists of three aspects: cohort selection (4 stars), comparability between RS and TEM groups (2 stars), and outcome assessment (3 stars), for a total of 9 stars. One to 3 stars were considered as low-quality studies, 4 to 6 stars were considered as moderate-quality studies, and 7 to 9 stars were considered as high-quality studies. Similarly, two investigators used the Cochrane Risk of Bias Assessment System (Review Manager 5.4 tool) to evaluate randomized controlled studies. The assessment system includes six aspects: random allocation method, allocation concealment, blinding, completeness of outcome data, selective reporting of study results, and other sources of bias. Each evaluation aspect can be rated at three levels: low risk, high risk, and unclear risk. Disagreements between two investigators on the evaluation of the literatures will be resolved by negotiation or by having a third-party expert review.
Statistical analysis
Different methods were used to conduct meta-analysis of effect sizes according to the type of effect size. For dichotomous variables, when sample size and outcome event rates were available in the original literature, the analysis could be performed directly with statistical software; when sample size and outcome event rates were not available in the original literature, the analysis could be performed with RR values and 95% confidence intervals. For continuous variables, the mean values and standard deviations were obtained from the literature for analysis; when continuous variables were presented as median and interquartile spacing, conversions could be performed with an online calculator provided by professor Tiejun Tong, Department of Mathematics, the University of Hong Kong, at the following links: hhttp://www.math.hkbu.edu.hk/~tongt/papers/median.html. I2 statistic was used to evaluate the heterogeneity between different literatures. When I2 > 50%, indicating obvious heterogeneity, a random effects model was used for data analysis. When I2 > 50%, indicating weak heterogeneity, a fixed-effect model was used for data analysis. This meta-analysis used Review Manager 5.4 and Stata MP 17. p values < 0.05 were considered statistically significant differences.
Results
Literature search results
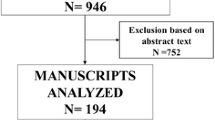
The search formula was developed to search in four English databases: 101 articles in PubMed, 54 articles in Web of Science, 29 articles in Cochrane library, and 13 articles in Embase. From other meta-analysis/reviews [10, 14] were 13, and the total number of literatures was 211. Duplicates were excluded, leaving 170 articles. According to the inclusion and exclusion criteria, 35 reviews, 5 conference abstracts, 54 inconsistent interventions, and 60 irrelevant studies were excluded by reading the titles and abstracts of the literatures. Sixteen full-text papers were read, and one full-text paper, one case report, and one Russian paper were excluded. Finally, this meta-analysis included 13 literatures, including 5 randomized controlled studies and 8 cohort studies.
Basic characteristics of the included studies
Thirteen papers, including 5 randomized controlled studies and 8 cohort studies, were included in this meta-analysis, and specific information is provided in Table 2.
Results of meta-analysis
Operative time
Seven papers [9, 11, 15,16,17,18,19] reported the operative time (minute) of both TEM and RS; there was significant heterogeneity (I2 = 93% > 50%, and Q test P < 0.05); no source of heterogeneity was found, so meta-analysis was performed based on random effects, and the results were as follows: the result after meta-combination was −97.14 (−115.81, −78.47), and there was a significant difference between TEM and RS (Z = 10.20, P < 0.05) (see Fig. 3A). On average, the operative time for TEM was 97.14 min shorter than that for RS, which means that the TEM surgical approach was effective in reducing the operative time Table 3.
Blood loss
Seven papers [9, 11, 15,16,17,18,19] reported surgical bleeding (ml) for both TEM and RS with significant heterogeneity (I2 = 99% > 50%,and Q test P < 0.05). By rejecting each article, no source of heterogeneity was found, so a meta-analysis was performed based on random effects, and the results were as follows: the effect size after meta-combination was −315.52 (−472.47, −158.57), and there was a significant difference between TEM and RS (Z = 3.94, P < 0.05) (see Fig. 3B).The surgical bleeding volume of TEM was 315.52 ml less than that of RS, which means that the TEM surgical approach was effective in reducing surgical bleeding volume.
Time of hospitalization
Six papers [9,10,11, 15, 18, 19] reported length of stay (days) for both TEM and RS procedures with significant heterogeneity (I2 = 82% > 50%, and Q test P < 0.05), and by excluding the literature one by one, Lezoche et al. [17] was found to be one of the sources of heterogeneity. Excluding Lezoche et al. [17], for meta-analysis again, the heterogeneity was significantly reduced (I2 = 51% > 50%, and Q test P > 0.05). So meta-analysis was performed based on random effects, and the results were as follows: the result after meta-merge was −8.82 (−10.62, −7.62), and there was a significant difference between TEM and RS (Z = 11.05, P < 0.05) (see Fig. 3C), and the length of stay was reduced by 8.82 days in case of TEM compared with RS, which means that the TEM surgical approach was significant in reducing the length of stay.
Postoperative complication
Twelve papers [9,10,11, 15,16,17,18,19,20,21,22,23] reported postoperative complications for both TEM and RS procedures with significant heterogeneity (I2 = 69% > 50%, and Q test P < 0.05), and no source of heterogeneity was found by article-by-article elimination, so a meta-analysis based on random effects was performed with the following results: the result after meta-combination was 0.35 (0.21, 0.59), and there was a significant difference between TEM and RS (Z = 3.98, P < 0.05) (see Fig. 4A), and the postoperative complication rate of TEM was lower than that of RS, showing that the TEM surgical approach was significantly effective in reducing the postoperative complication rate. Four papers [19, 21,22,23] reported the incidence of postoperative anastomotic stenosis for both TEM and RS procedures without significant heterogeneity (I2 = 0% < 50%, and Q test P > 0.05), so a meta-analysis based on fixed effects was performed with the following results: the result after meta-combination was 0.37 (0.09, 1.55), but there was no significant difference between TEM and RS (Z = 1.36, P > 0.05) (see Fig. 4B). Eight papers [9, 11, 16, 18,19,20,21,22,23] reported the incidence of postoperative suture dehiscence or anastomotic leak for both TEM and RS procedures without significant heterogeneity (I2 = 0% < 50%,and Q test P > 0.05), so a meta-analysis based on fixed effects was performed with the following results: the result after meta-combination was 0.57 (0.30, 1.06). However, there was no significant difference in the incidence of suture dehiscence or anastomotic leakage between the two surgical approaches (Z = 1.78, P > 0.05) (see Fig. 4C). Seven papers [9, 11, 16, 18, 19, 21, 23] reported the incidence of postoperative bleeding for both TEM and RS procedures. There was no significant heterogeneity (I2 = 0% < 50%, and Q test P > 0.05), so a meta-analysis based on fixed effects was performed with the following results: the result after meta-combination was 0.47 (0.22, 0.99), but there was no significant difference in the incidence of postoperative bleeding between the two surgical approaches (Z = 1.99, P = 0.05) (see Fig. 4D). Two papers [9, 23] reported the incidence of postoperative rectal pain after both TEM and RS procedures without significant heterogeneity (I2 = 0% < 50%, and Q test P > 0.05), so a meta-analysis based on fixed effects was performed, and the results were as follows: the result after meta-combination was 1.47 (1.11, 1.95). The incidence of rectal pain after TEM was 1.47 time higher than that of RS; there was a significantly different in the incidence of postoperative rectal pain between the two surgical approaches (Z = 2.70, P < 0.05) (see Fig. 4E). Three papers [9, 11, 23] reported the incidence of pneumonia after both TEM and RS procedures without significant heterogeneity (I2 = 0% < 50%, and Q test P > 0.05), so a meta-analysis based on fixed effects was performed, and the results were as follows: the result after meta-combination was 0.37 (0.10, 1.40), and the incidence of pneumonia after TEM was 0.37 times higher than the incidence of pneumonia after RS, but there was no significant difference in the incidence of postoperative pneumonia between the two surgical approaches (Z = 1.47, P > 0.05) (see Fig. 4F).
Temporary stoma
Three papers [17, 18, 23] reported temporary stoma for both TEM and RS, with significant heterogeneity (I2 = 76% > 50%, and Q test P < 0.05). By excluding each article, Simon P Bach (2021) [23] was found to be the source of heterogeneity. After excluding this article (I2 = 0% > 50%, and Q test P > 0.05), meta-analysis based on fixed effects was performed, and the results were as follows: the result after meta-merger was 0.05 (0.01, 0.20), and there was a significant difference between TEM and RS (Z = 4.35, P < 0.05) (see Fig. 5A); the temporary stoma of TEM was lower than that of RS.
Permanent stoma
Seven papers [10, 16,17,18,19, 22, 23] reported permanent stoma for both TEM and RS with significant heterogeneity (I2 = 61% > 50%,and Q test P < 0.05), and by excluding each article, Ptok was found to be the source of heterogeneity, and after excluding this article, I2 = 36% < 50%, and Q test P > 0.05, so meta-analysis based on fixed effects was performed: the result after meta-merge was 0.16 (0.08, 0.33), and there was a significant difference between TEM and RS (Z = 5.09, P < 0.05) (see Fig. 5B); the permanent stoma of TEM was lower than that of RS.
Perioperative mortality
Eleven papers [9,10,11,12,13,14,15,16, 18,19,20,21,22,23] reported perioperative mortality for both TEM and RS with no significant heterogeneity (I2 = 0% < 50%, and Q test P > 0.05), so a meta-analysis based on fixed effects was performed with the following results: the result after meta-combination was 0.26 (0.07, 0.93), and there was a significant difference between TEM and RS (Z = 2.08, P < 0.05) (see Fig. 6A). The perioperative mortality for TEM was 0.26 time greater than that for RS.
Local recurrence
Eleven publications [9, 11, 15,16,17,18,19,20,21,22,23,24] reported local recurrence rates for both TEM and RS procedures with significant heterogeneity (I2 = 55% > 50%, and Q test P < 0.05); by excluding the literature one by one, Stornes [24] was found to be the source of heterogeneity. By excluding this literature (I2 = 0% < 50%, and Q test P > 0.05), therefore based on fixed effects, a meta-analysis was performed, and the results were as follows: the result after meta-combination was 2.51 (1.53, 4.21), and there was a significant difference between TEM and RS (Z = 3.65, P < 0.05); the local recurrence rate for TEM was 2.51 times higher than the local recurrence rate for RS. Figure 6 is a subgroup analysis based on whether neoadjuvant treatment was performed. Intervention of three papers [14, 17, 22] was TEM with neoadjuvant therapy and RS, and the other literature[16] was neoadjuvant therapy for both TEM and RS. The results showed that with neoadjuvant therapy, there was no significant difference in local recurrence rate for both procedures (Z = 1.23, P > 0.05). While without neoadjuvant therapy, TEM was significantly higher than RS regarding the local recurrence rate (Z = 2.98, P < 0.05).
Distant metastasis
Eleven papers [9, 11, 15,16,17,18,19,20,21, 23, 24] reported the incidence of distant metastasis for both TEM and RS surgical procedures without significant heterogeneity (I2 = 0% < 50%, and Q test P > 0.05), so a meta-analysis based on fixed effects was performed with the following results: the result after meta-combination was 0.59 (0.34, 1.02), and the distant metastasis for TEM was lower than that for RS. However, there was no significant difference in distant metastasis between the two surgical approaches of TEM and RS (see Fig. 6C).
Overall recurrence
Eight papers [10, 11, 15, 16, 18,19,20,21] reported overall recurrence after both TEM and RS procedures without significant heterogeneity (I2 = 0% < 50%, and Q test P > 0.05), so a meta-analysis based on fixed effects was performed, and the results were as follows: the result after meta-combination was 1.49 (0.96, 2.31), and the overall recurrence rate for TEM was 1.49 times higher than that for RS, but there was no significant difference in overall recurrence between the two surgical modalities of TEM and RS (see Fig. 6D).
Overall survival
Ten publications [9, 11, 15, 16, 18,19,20,21, 23, 24] reported overall survival after both TEM and RS procedures. And the effect size was the RR value and 95% CI of overall survival. There was significant heterogeneity (I2 = 55% > 50%, and Q test P < 0.05). No source of heterogeneity was found by article-by-article elimination, so a meta-analysis was performed based on random effects, and the results were as follows: the result after meta-combination was 0.88 (0.74, 1.00), and the effect size was significant difference (Z = 12.92, P < 0.05) (see Fig. 7A). The overall survival rate for TEM was lower than the overall survival rate for RS. The results of the subgroup analysis showed that the effect values were hazard ratio and 95% confidence interval; TEM reduced overall survival (HR = 1.314 (95% CI 0.931, 1.697)) with significant difference (Z = 6.721, P = 0.000 < 0.05); for the T2 early-stage cancer without neoadjuvant treatment subgroup [15, 21, 24], the adverse effect of TEM on overall survival was more significant (HR = 1.847 (95% CI 0.994, 2.701)), with statistically significant (Z = 4.242, P = 0.000 < 0.05). However, for the T2 early-stage cancer, combined with neoadjuvant therapy subgroup [15, 18] (HR = 1.286 (95% CI −0.114, 2.685)), TEM did not significantly reduce overall survival (see Fig. 7B).
Disease-free survival
Three papers [11, 21, 23] reported disease-free survival after both TEM and RS procedures. The effect value was RR values for disease-free survival and 95% CI. Without no significant heterogeneity (I2 = 0% < 50%, and Q test P > 0.05), meta-analysis-based fixed effects was performed. The results were as follows: the result after meta-combination was 1.08 (0.97, 1.19), and the effect size was significant (Z = 19.31, P < 0.05) (see Fig. 7C). Disease-free survival surgery for TEM was 1.08 times higher than disease-free survival surgery for RS; that is, both TEM and RS surgical modalities differed significantly in terms of disease-free survival.
Disease-specific survival
Three papers [16, 18, 19] reported disease-specific survival after both TEM and RS; effect sizes were RR values and 95% CI for overall survival without significant heterogeneity (I2 = 0% < 50%, and Q test P > 0.05), so meta-analysis based on fixed effects was performed with the following results: the result after meta-combination were 0.74 (0, 091.54), Z = 1.756 P = 0.079 > 0.05 (see Fig. 7D), and the disease-free survival surgery of TEM was 0.74 times higher than the surgical disease-free survival of RS, but there was no significant difference for both TEM and RS.
Discussion
Based on the results of this meta-analysis, TEM was associated with a higher risk of local recurrence compared with RS and was superior in terms of disease-free survival, reduced perioperative mortality, temporary stoma, permanent stoma, and postoperative complications; however, the overall survival of TEM was lower than RS, and the rectal pain rate was higher than RS. In addition, TEM was effective in reducing operative time, postoperative bleeding, and time of hospitalization. There was no statistically significant difference between the two surgical approaches in terms of distant metastasis, overall recurrence, disease-specific survival, and the incidence of postoperative complications such as suture dehiscence or anastomotic leakage, postoperative bleeding, pneumonia, and anastomotic stenosis. Our findings demonstrate that TEM is one of the effective and safe alternatives to RS, and more high-quality studies are needed to prove this.
In clinical practice, local recurrence is a complication to avoid with regard to the choice of TEM. We concluded that the local recurrence rate was higher in patients treated with TEM compared with RS, while the overall recurrence rate was no significantly different. After rigorous and careful screening, patients who experienced recurrence can undergo salvage surgery again. However, 5-year overall survival rate was 50% (95% CI, 30–74%), and re-recurrence-free survival was 47% (95% CI, 25–68%). Oncologic outcomes are poor[26]. Several studies have come to the same conclusion [14, 27]. Possible reasons for high local recurrence after TEM are as follows: high-grade pathological grading, distance of the incision margin from the margin of the primary focus, T-staging, and occult lymph node metastasis. The study of T Junginger [28] showed that the probability of local recurrence was 11% for low-grade pathology and 25% for high-grade pathology and 10% and 38% for local recurrence when the pathology was both low risks, and the distance of the cut edge from the edge of the primary focus was > 1 mm and < 1 mm, respectively. The study of Morino [29] showed that sm stage, T stage, and tumor grade were independent predictors of local recurrence in multivariate analysis. When no intervention was taken for local recurrence, the median survival of patients was at 8 months [30]. The median survival of patients with rectal cancer recurrence is 47%, 38%, and 35% at 5, 10, and 15 years, respectively [31, 32]. Although salvage RS can remove recurrent lesions after local recurrence in patients who have undergone TEM, most patients do not achieve complete resection [33]. Therefore, RS has a better outcome in terms of survival. TEM combined with neoadjuvant therapy is similar to RS in terms of local recurrence and survival in early-stage rectal cancer (T2), and TEM is one of the alternatives to RS in appropriately selected patients [34].
In terms of quality of life, we concluded that the rate of stoma is significantly higher in both temporary and permanent stoma in RS than in TEM. Postoperative stoma can significantly impair physiological function and reduce quality of life [35]. The extensive and invasive nature of RS not only significantly impairs patients’ quality of life and social functioning, but also promotes health anxiety and affects family social relationships. Simon P Bach [23] has shown that RS is associated with adverse symptoms, such as changes in bowel habits, urinary incontinence, bowel dysfunction, and sexual dysfunction, which may have a negative impact on daily life. On the contrary, TEM is a local excisional surgery, which can preserve the autonomic nerves in the pelvic cavity, thus preserving the normal urinary, bowel, and sexual functions; at the same time, it protects the anal sphincter muscle, thus preserving the anal function and reducing the probability of stoma. Our study found that the TEM surgical approach significantly reduced the overall incidence of postoperative complications, but there were no significant differences between two surgical approaches. One study [14] concluded that TEM was more advantageous in terms of overall postoperative complication rates and that the TEM reduced both anastomotic stenosis and postoperative bleeding. The reason for the different results may be that a randomized controlled study included in our study [23] intervention was that short-term radiotherapy combined with TEM had a positive impact on the occurrence of postoperative complications. Given that TEM can reduce the incidence of postoperative complications and improve quality of life, this surgical approach is suitable for elderly patients with severe diseases, poor physical fitness, and short life expectancy, and the TEM surgical approach can significantly reduce the operative time, intraoperative bleeding, and length of hospital stay and reduce the financial burden on patients. The results of this study showed that the perioperative mortality was lower with the TEM approach, which was 0.26 times lower than that of RS. E.J.R. De Graaf [16] study showed that the perioperative mortality rate for RS ranged from 3.3 to 25.8%. Also, some studies [36, 37] showed that TEM has been a safer surgical procedure with a clear view of the surgical field, clear identification of tissue structures, precise separation of the tumor, less stoma, and fewer complications than RS. In terms of long-term survival, we concluded that the TEM surgical approach reduced overall survival, and the Xiaoyu Xiong [14] found that similar results were obtained in the study, where the adverse effect of TEM on overall survival was more pronounced when subgroup analysis was performed with T2 staging and without neoadjuvant therapy; when neoadjuvant therapy was performed to patients with early-stage rectal cancer, there was no significant difference between the two surgical approaches in terms of overall survival. The results suggest that for rectal cancer patients with T2 stage, neoadjuvant therapy improves overall survival. Intervention of Simon P Bach [23] for early-stage rectal cancer was short-term radiotherapy combined with TEM and TME, and the results showed no significant difference in overall survival between the two interventions over a mean follow-up period of 4.28 years. The results demonstrate that neoadjuvant therapy improves the overall survival of TEM surgery. Our study found that in terms of disease-free survival, although there was a statistically significant difference between TEM and RS, the RR value was only 1.08. This indicated that there was no significant difference between TEM and RS. Tan S [38]. showed that there was no significant difference between local resection and radical resection in terms of disease-free survival. In this respect, TEM or combined neoadjuvant therapy is an alternative option for the treatment of early rectal cancer. Several studies_[39, 40] found that 89% of T1 and 72% of T2 of rectal cancer patients experienced unnecessary RS procedure, respectively, resulting in a significant increase in the incidence of adverse events such as urinary incontinence, sexual dysfunction, bowel dysfunction, anastomotic leak, and permanent or temporary stoma, which seriously affects the quality of life of patients. Endoscopic and pathologic examination for diagnosis and pathologic grading, endoscopic ultrasound to determine the depth of infiltration, MRI and CT to determine the presence of distant metastases, and screening of patients eligible for surgery are top priorities. According to the 2018 NCCN Guidelines [41], the surgical approach of local excision is recommended for patients with T1 and N0 with early-stage rectal cancer and tumor diameter < 3 cm, intermediate to advanced pathological grade, and tumor invasion < 30% of the circumference of the bowel, and RS is recommended for patients with T2 rectal cancer; T1 rectal cancer presenting with positive margins, lymphovascular infiltration, and poorly differentiated or infiltrated to the lower third of the submucosa (sm3 level), RS is recommended [42, 43]. The use of TEM for rectal cancer patients with T1 N0 and without high-risk pathologic features has received wide acceptance. In these patients, the local recurrence and overall survival rates of TEM are comparable to those of RS and have a much lower impact on quality of life [44]. S. V. Chernyshovf [45] found that the 3-year disease-free survival rates for both TEM and RS were 92% and 96%, respectively, indicating that TEM is a safe and effective surgical procedure for the treatment of early rectal cancer in T1. Other studies [34, 46] found that for patients with T2-staged rectal cancer, local excision alone significantly reduces patient disease-free survival; and when neoadjuvant therapy is combined with local excision, the oncologic outcome can be compared to that of RS. Neoadjuvant therapy combined with local excision [47,48,49] can be applied to T2 and even to T3 and T4, and the neoadjuvant therapy can reduce the tumor stage and improve the organ preservation rate of the rectum in order to improve the quality of life while obtaining good tumor outcome. Neoadjuvant chemotherapy has been shown to improve local recurrence rates and reduce toxicity. However, it is unclear that the impact of neoadjuvant therapy on survival in patients with early-stage rectal cancer who undergo radical surgery adhering to total mesorectal excision [50].
Meanwhile, this meta-analysis has some limitations. Firstly, most of the included literatures were non-randomized controlled studies, and the reliability of the conclusions was low; secondly, the inclusion criteria of study subjects were different among different literatures, and there was a strong heterogeneity; third the outcome index measures of different literatures had a large heterogeneity, which might lead to less reliable conclusions. In the future, we will conduct prospective, multicenter, randomized controlled studies with large samples to provide a reliable basis for clinical decision-making.
Conclusion
In conclusion, limited evidence suggests that TEM can shorten operative time and hospital stay, reduce intraoperative bleeding, decrease the incidence of postoperative complications and perioperative mortality, reduce stoma rates, and improve quality of life and is a safe surgical alternative to RS in selected patients. Neoadjuvant therapy in combination with TEM is promising to improve the higher local recurrence rate of TEM and long-term survival and could be tried in patients with advanced rectal cancer.
Data Availability
Data available on request from the authors.
References
Bray F et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Zhang X et al (2019) Short- and long-term outcomes of transanal versus laparoscopic total mesorectal excision for mid-to-low rectal cancer: a meta-analysis. Surg Endosc 33(3):972–985
Health NIFC (2014) Excellence, colorectal cancer: the diagnosis and management of colorectal cancer. NICE
Boyle J et al (2018) National Bowel Cancer Audit Annual Report 2018. NHS
Snijders HS et al (2012) Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur J Surg Oncol 38(11):1013–1019
Gilbert A et al (2015) Systematic review of radiation therapy toxicity reporting in randomized controlled trials of rectal cancer: a comparison of patient-reported outcomes and clinician toxicity reporting. Int J Radiat Oncol Biol Phys 92(3):555–567
Wiltink LM et al (2016) A comprehensive longitudinal overview of health-related quality of life and symptoms after treatment for rectal cancer in the TME trial. Acta Oncol 55(4):502–508
Zolciak A et al (2006) Abdominoperineal resection or anterior resection for rectal cancer: patient preferences before and after treatment. Colorectal Dis 8(7):575–580
Chen YY et al (2013) Transanal endoscopic microsurgery versus laparoscopic lower anterior resection for the treatment of T1–2 rectal cancers. Hepatogastroenterology 60(124):727–732
Langer C et al (2003) Surgical cure for early rectal carcinoma and large adenoma: transanal endoscopic microsurgery (using ultrasound or electrosurgery) compared to conventional local and radical resection. Int J Colorectal Dis 18(3):222–229
Palma P et al (2009) Local excision of early rectal cancer: is transanal endoscopic microsurgery an alternative to radical surgery? Rev Esp Enferm Dig 101(3):172–178
Kawaguti FS et al (2014) Endoscopic submucosal dissection versus transanal endoscopic microsurgery for the treatment of early rectal cancer. Surg Endosc 28(4):1173–1179
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Xiong X et al (2021) Can transanal endoscopic microsurgery effectively treat T1 or T2 rectal cancer? A systematic review and meta-analysis. Surg Oncol 37:101561
Allaix ME et al (2012) Transanal endoscopic microsurgery vs. laparoscopic total mesorectal excision for T2N0 rectal cancer. J Gastrointest Surg 16(12):2280–2287
De Graaf EJ et al (2009) Transanal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol 35(12):1280–1285
Lezoche G et al (2008) A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg Endosc 22(2):352–358
Lezoche E et al (2012) Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 99(9):1211–1218
Winde G et al (1996) Surgical cure for early rectal carcinomas (T1). Transanal endoscopic microsurgery vs. anterior resection. Dis Colon Rectum 39(9):969–76
Heintz A, Mörschel M, Junginger T (1998) Comparison of results after transanal endoscopic microsurgery and radical resection for T1 carcinoma of the rectum. Surg Endosc 12(9):1145–1148
Lee W et al (2003) Transanal endoscopic microsurgery and radical surgery for T1 and T2 rectal cancer. Surg Endosc 17(8):1283–1287
Ptok H et al (2007) Oncological outcome of local vs radical resection of low-risk pT1 rectal cancer. Arch Surg 142(7):649–655
Bach SP et al (2021) Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): a randomised, open-label feasibility study. Lancet Gastroenterol Hepatol 6(2):92–105
Stornes T et al (2016) National early rectal cancer treatment revisited. Dis Colon Rectum 59(7):623–629
Stornes T et al (2016) National early rectal cancer treatment revisited. Dis Colon Rectum 59(5):e204–e205
Bikhchandani J et al (2015) Outcomes of salvage surgery for cure in patients with locally recurrent disease after local excision of rectal cancer. Dis Colon Rectum 58(3):283–287
Veereman G et al (2017) Systematic review and meta-analysis of local resection or transanal endoscopic microsurgery versus radical resection in stage i rectal cancer: a real standard? Crit Rev Oncol Hematol 114:43–52
Junginger T et al (2017) Long-term results of transanal endoscopic microsurgery after endoscopic polypectomy of malignant rectal adenoma. Tech Coloproctol 21(3):225–232
Morino M et al (2011) Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc 25(11):3683–3690
Palmer G et al (2007) A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol 14(2):447–454
Heriot AG et al (2006) Surgery for local recurrence of rectal cancer. Colorectal Dis 8(9):733–747
Cyr DP et al (2020) Long-term outcomes following salvage surgery for locally recurrent rectal cancer: a 15-year follow-up study. Eur J Surg Oncol 46(6):1131–1137
Stipa F, Giaccaglia V, Burza A (2012) Management and outcome of local recurrence following transanal endoscopic microsurgery for rectal cancer. Dis Colon Rectum 55(3):262–269
Garcia-Aguilar J et al (2015) Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 16(15):1537–1546
Neuberger L et al (2022) A new stoma for an older person-An association with quality of life and physical function: a systematic review. J Am Geriatr Soc 70(8):2415–2425
Middleton PF, Sutherland LM, Maddern GJ (2005) Transanal endoscopic microsurgery: a systematic review. Dis Colon Rectum 48(2):270–284
Doornebosch PG et al (2007) Quality of life after transanal endoscopic microsurgery and total mesorectal excision in early rectal cancer. Colorectal Dis 9(6):553–558
Tan S et al (2022) Local resection versus radical resection for early-stage rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 37(7):1467–1483
Salinas HM et al (2011) Determining the need for radical surgery in patients with T1 rectal cancer. Arch Surg 146(5):540–543
Stewart DB, Dietz DW (2007) Total mesorectal excision: what are we doing? Clin Colon Rectal Surg 20(3):190–202
Benson AB et al (2018) Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 16(7):874–901
Yamamoto S et al (2004) The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology 51(58):998–1000
Nascimbeni R et al (2002) Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 45(2):200–206
Allaix ME, Arezzo A, Morino M (2016) Transanal endoscopic microsurgery for rectal cancer: T1 and beyond? An evidence-based review Surg Endosc 30(11):4841–4852
Chernyshov SV et al (2022) Results of total mesorectal excision and transanal endoscopic microsurgery for rectal adenocarcinoma with submucosal invasion. Khirurgiia (Mosk) 4:34–41
Bhangu A et al (2013) Survival outcome of local excision versus radical resection of colon or rectal carcinoma: a surveillance, epidemiology, and end results (SEER) population-based study. Ann Surg 258(4):563–9, discussion 569–71
Rullier E et al (2017) Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 390(10093):469–479
Kennecke HF et al (2022) Neoadjuvant chemotherapy, excision, and observation for early rectal cancer: the phase II NEO Trial (CCTG CO.28) primary end point results. J Clin Oncol JCO2200184
Bosset JF et al (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355(11):1114–1123
Phillips JG, Hong TS, Ryan DP (2012) Multidisciplinary management of early-stage rectal cancer. J Natl Compr Canc Netw 10(12):1577–1585
Funding
This study was supported by the Jianghan Talent Funding (No. 02.05.22030036). We thank the National Natural Science Foundation of China (81871962) and the Fund for Fostering Young Scholars of Peking University Health Science Center (BMU2018PYB014) for providing support.
Author information
Authors and Affiliations
Contributions
The following authors have substantial contributions to the conception and design of the study, the acquisition of data, or analysis and interpretation of data: Wei Li, Xing Xing Xiang, Chen Jun Cai, and Hong Da Wang. Wei Li led the conceptualization and provided valuable supervision and oversight the project. Drafting and revising the article were done by Wei Li, Xing Xing Xiang, Chen Jun Cai, and Hong Da Wang. Final approval of the version to be published was done by Wei Li and Xing Xing Xiang. Wei Li and Xing Xing Xiang drafted and prepared the main manuscript text.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
No informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, W., Xiang, X.X., Da Wang, H. et al. Transanal endoscopic microsurgery versus radical resection for early-stage rectal cancer: a systematic review and meta- analysis. Int J Colorectal Dis 38, 49 (2023). https://doi.org/10.1007/s00384-023-04341-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s00384-023-04341-9